Slideshow
advertisement

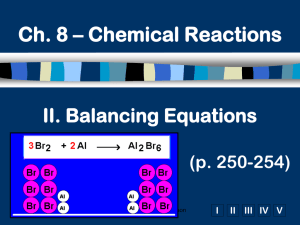
Ch. 8 – Chemical Reactions I. Intro to Reactions II. Balancing Equations (p. 241 – 254) A.Signs of a Chemical Reaction Evolution of heat and light - large amounts relative to physical Formation of a gas - effervescence, new smell Formation of a precipitate - obvious solid Color change - sometimes... B.Law of Conservation of Mass mass is neither created nor destroyed in a chemical reaction total mass stays the same atoms can only rearrange 4H 36 g 2O 4H 2O 4g 32 g C. Chemical Equations A+B C+D REACTANTS PRODUCTS C. Chemical Equations p. 246 D. Writing Equations 2H2(g) + O2(g) 2H2O(g) Use symbols to show: How many? - coefficient Of what? - chemical formula In what state? - physical state Remember the diatomic elements. D. Writing Equations Aluminum reacts with aqueous copper(II) chloride to produce copper and aqueous aluminum chloride. 2Al(s) + 3CuCl2(aq) 3Cu(s) + 2AlCl3(aq) Zn(s) + 2HCl(aq) ZnCl2(aq) + H2(g) D. Writing Equations Coefficients can mean: individual atom = “atom” covalent substance = “molecule” ionic substance = “formula unit” 3CO2 3 molecules 2Mg 2 atoms 4MgO 4 formula units REACTING MOLAR RATIOS!! E. Balancing Steps 1. Write the unbalanced equation. 2. Count atoms on each side. 3. Add coefficients to make #s equal. Coefficient subscript = # of atoms 4. Reduce coefficients to lowest possible ratio, if necessary. 5. Double check atom balance!!! E. Balancing Example Aluminum and copper(II) chloride react to form copper and aluminum chloride. 2 Al + 3 CuCl2 3 Cu + 2 AlCl3 E. Balancing - Helpful Tips Balance one element at a time. Update ALL atom counts after adding a coefficient. If an element appears more than once per side, balance it last. Balance polyatomic ions as single units. “1 SO4” instead of “1 S” and “4 O” E. Balancing Examples Fe + H2O --> Na + O2 --> CaO + S+ Fe3O4 + Na2O H2O --> O2 --> H2 SO3 Ca(OH)2 F. Algebraic Method equation: Al2(SO4)3 + CaCl2 --> AlCl3 + CaSO4 add letters: a Al2(SO4)3 + b CaCl2 --> c AlCl3 + d CaSO4 list atoms: Al S O Ca Cl assign variables: 2a = c 3a = d 12a = 4d b=d 2b = 3c F. Algebraic Method calculate: a=1 c=2 d=3 b=3 finish with coefficients: 1Al2(SO4)3 + 3CaCl2 --> 2AlCl3 + 3CaSO4 CHECK YOUR WORK!! F. Algebraic Method BCl3 + P4 + H2 BP + HCl