Carbohydrates - A Level Notes
advertisement

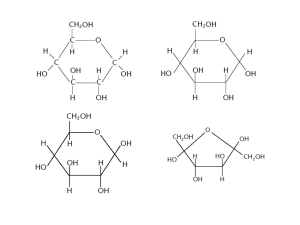
Carbohydrates • Energy storage and supply (in respiration) • Structure (e.g. cellulose in plant cell walls) • Contain carbon and hydrogen • General formula: Cx(H2O)y • Polymer = polysaccharide • Disaccharides and monosaccharide’s both dissolve in water to give a sweet tasting solution • Monomer = monosaccharide. Cn(H2O)n, 1 sugar unit. • 3 Carbon atoms = Triose, e.g. glyceraldehyde • 5 Carbon atoms = Pentose, e.g. ribose • 6 Carbon atoms = hexose, e.g. glucose Glucose Isomers are molecules with the same molecular formula, sometimes even the same structural formula, but a different special arrangement. Alpha glucose in the ring has the H atom Above the rest of the ring, with the OH group pointing down. Beta glucose in the ring has the H atom Below the ring, and the OH group pointing up. Alpha and Beta glucose are isomers because they have the same molecular formula, but the special arrangement of the hydroxyl group when the ring is formed is different. Glucose is an energy source because it can be broken down into smaller molecules of water and carbon dioxide in respiration, releasing energy for ATP. Glucose is a polar molecule so it can form hydrogen bonds and dissolves in water. Glucose condenses to form di and polysaccharides because of the hydroxyl groups. Other monosaccharides • Ribose – present in RNA and ATP • Deoxyribose – Part of DNA, information molecule • Fructose – Fruit sugars, makes for a sweeter taste. Fructose + glucose -> sucrose • Galactose – Galactose +glucose -> lactose Formation of a Disaccharide Disaccharides are formed from two monosaccharides in a condensation reaction. The reverse is a hydrolysis reaction. An enzyme is required to make both of these reactions take place. The free OH groups, both downward facing in Alpha molecules, join, creating a spare water molecule, and creating a glycosidic bond. A bond between the 1st carbon in one monosaccharide and the 4th carbon in the second, both alpha monosaccharides, creates an alpha 1, 4 glycosidic bond / linkage. Polysaccharides Polysaccharides are formed when many monosaccharides are joined together in a condensation reaction to form a macromolecule (polymer). Cells cannot store glucose because it is soluble and will dissolve, affecting the water potential of the cell, so instead plants and animals store glucose as starch (plants) and glycogen (animals). Both are polymers of alpha lucose and act as an energy store. Storage is an advantage because it is more compact than glucose, inert, insoluble in water, can be quickly broken down into alpha glucose which can be respired. Starch Starch is made of 80% amylopectin and 20% amylose. Amylose is a coiled, unbranched, spiralling helical structure, because of its alpha 1, 4 glycosidic bonds. Amylopectin has a branched shape because of its main chain of alpha 1, 4 glycosidic bonds, and then at the branches alpha 1, 6 bonds. These mean it has more ends for the enzymes to act on for rapid energy release when necessary. Glycogen Glycogen also has a highly branched polymer of alpha glucose, with long chains linked by alpha 1, 4 glycosidic bonds and with many, short branches created by alpha 1, 6 glycosidic bonds. Because it has more and shorter branches, it is even easier for the enzymes to act. It is stored mainly in muscle and liver cells. • Muscle – For easy release when muscle fatigue sets in, for more movement. • Liver – when blood glucose levels are too high, glucagon is released, so that the excess glucose in the blood can be sieved out and stored in the liver as glycogen. Cellulose Cellulose is a structural polysaccharide. It is a polymer of beta glucose. Cellulose is a linear, unbranched chain, because the beta monosaccharides need to be rotated 180 degrees before they can be glycosidically linked, so the alternately rotated bonds mean it remains linear. The chains form fibrils and hydrogen bonds form between adjacent fibrils, forming bundles called fibres. Fibres have high tensile strength and they lay in a mesh to create a strong cell wall. Cellulose is bonded by beta 1, 4 glycosidic bonds.