Organic Analysis

LABORATORY ANALYSIS
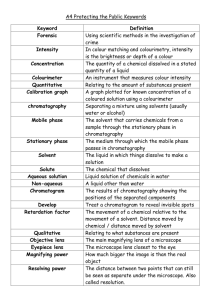
Forensic Science
Elements and Compounds
A. Matter - anything that has mass & takes up space
B. Element - cannot be broken down into simpler substances by chemical means
C. Periodic table - chart of elements arranged in a systematic fashion
D. Atom - smallest particle of an element that can exist and still retain its identity as that element
Mixtures & Compounds
Mixture – Two or more substances that are mixed together, but not chemically combined.
Examples of mixtures ...
Air – mixture of gases
Bowl of cereal – mixture of cereal and milk
Soda pop – mixture of soda syrup, water, and CO 2 gas
Fog –water suspended in air
Kool-Aid – mixture of water, sugar, and flavor crystals
Compounds – Two or more elements that are chemically combined.
Examples of compounds ...
Salt –Sodium and chlorine combined chemically
Water –Hydrogen and oxygen combined chemically
Carbon Dioxide – Carbon and oxygen combined chemically
Solutions
Solutions are mixtures in which one substance is dissolved in another.
Solutions have two parts: solute and solvent
The solute is the substance that is dissolved.
The solvent is the substance that does the dissolving
Identify the solute and solvent in each solution ...
Solution Solute Solvent
Lemonade
Soda pop
Ocean water
Solubility A measure of how much of a given substance will dissolve in a liquid.
A substance that does not dissolve in water is called insoluble .
A substance that does dissolve in water is called soluble .
The periodic table is a listing of all elements by increasing atomic number.
The vertical columns are called groups.
There are 18 groups
The horizontal rows are called periods.
There are 7 periods.
The periodic table can be separated into metals, nonmetals, and metalloids.
Metals are shiny, malleable, ductile, and good conductors of heat and electricity.
Nonmetals are not shiny, malleable, ductile, or good conductors of heat and electricity.
Physical states
Substances change from one state to another
Phase - a uniform piece of matter, different phases are separated by definite visible boundaries
There are four phases of matter: solid, liquid, gas, and plasma.
Phase Changes – Physical Changes
Evaporation = Liquid -> Gas
Condensation = Gas -> Liquid
Melting = Solid -> Liquid
Freezing = Liquid -> Solid
Sublimation = Solid -> Gas
Solids
particles vibrate but can’t move around
fixed shape
fixed volume
incompressible
Liquids
particles can move around but are still close together
variable shape
fixed volume
Virtually incompressible
Gases
particles can separate and move throughout container
variable shape
variable volume
Easily compressed
Vapor - gaseous state of a substance that is a liquid or solid at room temperature
Plasma
particles collide with enough energy to break into charged particles (+/-)
gas-like, variable shape & volume
stars, fluorescent light bulbs, TV tubes
Four States of Matter
Selecting an Analytical Technique
Need to know whether substance is organic or inorganic
A. Organic - substance composed of carbon and hydrogen
B. Inorganic - CO
2 lack carbon and all substances that
Need to consider the need for qualitative and quantitative determination
Organic Analysis
Spectrophotometry
Chromatography
Gas Chromatography (GC)
High – Performance Liquid Chromatography
(HPLC)
Thin – Layer Chromatography (TLC)
Electrophoresis
Spectrophotometry
An analytical method for identifying a substance by its selective absorption of different wavelengths of light
Most applicable to organic analysis
Optimum utilization requires that a material be in relatively pure state
Chromatography
Organic mixtures are separated into their components by their attraction to a stationary phase while being propelled by a moving phase.
Useful technique for purifying substances
1 st observed in 1803 by William Henry (Henry’s Law)
One phase is always made to move continuously in one direction over a stationary or fixed phase
It’s like a race between chemical compounds.
At the beginning, all substances are mixed together.
As the race progresses, those that have preference for the moving phase will move faster and pull ahead of others.
At the end, all the substances are separated.
Gas Chromatography (GC)
separates mixtures on the basis of their distribution between a stationary liquid phase and a moving gas phase
used widely because of its ability to resolve a highly complex mixture into its components within a time period usually measured in minutes
is very sensitive
sample must be vaporized and passed through heated tube
High-Performance Liquid Chromatography
(HPLC)
Different types of stationary phases (usually nonaqueous) with a liquid moving phase
can perform process at room temperature
used for organic explosives and drugs that are heat sensitive
Thin-Layer Chromatography (TLC)
incorporates solid stationary phase & liquid moving phase
because most compounds are colorless, uses UV light to reveal those that fluoresce
cannot by itself provide absolute identification; has to be used in conjunction with other procedures to prove absolute identity
powerful tool for solving analytical problems
rapid and sensitive
minimal cost and space requirements
Electrophoresis
related to TLC in that it separates materials according to their migration rates on solid phase
uses electrical current instead of moving liquid phase
characterization of proteins, enzymes, and DNA
Examples of Chromatography
Liquid Chromatography
Used to identify unknown plant pigments & other compounds.
Thin-Layer Chromatography
Uses thin plastic or glass trays to identify the composition of pigments, chemicals, and other unknown substances.
Gas Chromatography
Used to determine the chemical composition of unknown substances, such as the different compounds in gasoline shown by each separate peak in the graph below.
Paper Chromatography
Can be used to separate the components of inks, dyes, plant compounds (chlorophyll), make-up, and many other substances
Paper Chromatography Lab
Obtain the supplies you’ll need.
1 large beaker (or plastic cup)
Pencil
1 small beaker (or plastic cup) filled with water
4 pieces of filter paper
4 black markers for testing
4 small pieces of masking tape
Pencil (to attach to the top of the filter paper)
Permanent marker
Timer
Filter
Paper
Ink
Mark
Tape – Label with marker
Write the pen number on a piece of masking tape with a permanent marker and place it at the top of the strip.
Choose one of the testing markers and draw a thick line near the bottom of the filter paper - about ¼ inch from the bottom.
Pour a small amount of water into the large cup and then hang the paper strip in the cup. Make sure the ink line does not touch the water – only the bottom of the filter paper.
Allow the water to move up the paper for 5 minutes and then remove the strip from the water. Hang it on the side of the table to dry.
Follow these directions to test the other pens.
Complete the chart on your worksheet and then answer the questions.
Questions:
What colors did your group observe in each of the black ink samples?
Do the colors occur in the same order on all the samples?
Explain.
Did some ink samples not work? Why?
Chromatography Challenge
Work with your group to identify the pens used for each of the
“Mystery Marks”.
1 st – Test each of the Mystery Mark strips using the procedure from yesterday.
2 nd – Compare your strips to the strips hanging in the classroom.
3 rd – Write the number of the pen that you think matches each of the mystery marks in the space on your worksheet.
4 th – Have your answers checked by the teacher. Keep trying until you are able to identify all 6 pens!
Pen A matches # _____ Pen D matches # _____
Pen B matches # _____
Pen C matches # _____
Pen E matches # _____
Pen F matches # _____
Inorganic Analysis
Carbon does not appear among earth’s most abundant elements.
Inorganics are also encountered as physical evidence
Metals in tools, coins, weapons, scrapings
Pigments in paints and dyes
Explosive formulations
Poisons
For ID & comparison of physical evidence
The Emission Spectrum of Elements
Elements selectively absorb and emit light
When the light passes through a prism, it is separated into its component colors or frequencies
(emission spectrum).
Types of emission spectrums
Continuous spectrum
shows a continuous band of colors all blending into one another
Ex – sunlight or light from incandescent bulb passes through a prism
Types of Emission Spectrum cont.
Line spectrum
1. shows a series of lines separated by black areas
2. Each line represents a definite wavelength or frequency
3. Ex – light from a sodium lamp or mercury arc lamp
Emission Spectrograph
instrument used to obtain and record the line spectra of elements
Requires:
1) a means for vaporizing and exciting the atoms of elements so that they emit light
2)
3) a means for separating light into its component frequencies a means for recording the resultant spectrum
Uses in Forensics: Rapid comparison of the elemental composition of two or more specimens
Inductively Coupled Plasma (ICP)
Emission Spectrometry
Identifies and measures elements through light emitted by excited atoms
Uses hot plasma torch instead of electrical arc to excite atoms
Has been applied in the area of identification and characterization of mutilated bullets and glass fragments
Atomic Absorption Spectrophotometer
When an atom is vaporized, it will absorb many of the same frequencies of light that it emits in an excited state.
In this technique, the specimen is heated to a temperature that is hot enough to vaporize its atoms while leaving a substantial number of atoms in an unexcited state.
Provides a determination of an element’s concentration
Useful in detecting trace amounts of elements
Drawbacks:
only one element at a time
each time the proper lamp has to be selected to match the particular element under investigation
Origin of Emission & Absorption Spectra
Subatomic particles
Proton – positive electrical charge; in nucleus
Neutron – neutral particle; in nucleus
Electron – negative charge; outside the nucleus
# of protons = # of electrons in a neutral atom
Electrons and Energy
Electrons move around the nucleus and are confined to specific electron orbitals or energy levels.
Atoms are most stable when all of the electrons are in the lowest possible energy orbitals.
When the atom absorbs energy (heat or light), its electrons are pushed into higher energy orbitals.
(excited state)
Only a definite amount of energy can be absorbed when moving an electron from one level to another.
Elements are selective in the frequency of light they absorb.
Energy levels determine the selectivity.
If atoms are exposed to intense heat, energy is generated to push electrons into higher unoccupied energy levels.
Normally, an electron does not remain in this excited state for long, but falls back to its original energy level. As it falls, it releases energy in the form of light.
Because each element has its own characteristic set of energy levels, each will emit a unique set of frequency values providing a “picture” of the energy levels that surround the nucleus of each element.
Neutron Activation Analysis
Atoms of the same element have the same number of protons.
They do not always have the same # of neutrons.
Atomic mass - the # of protons + # of neutrons
Isotopes - atoms with the same # of protons but different # of neutrons
Most elements have two or more isotopes, and most are stable.
Isotopes that are unstable and decompose are considered radioactive.
Radioactivity
Atoms having the same number of protons but differing solely in the number of neutrons are called isotopes.
Most elements have two or more isotopes and most are stable.
Isotopes that are unstable and decompose are considered to be radioactive.
Radioactivity is the emission of radiation that accompanies the spontaneous disintegration of unstable nuclei.
Radioactivity
Types
Alpha particles (
)– composed of a helium nucleus; positive charge 4
He
2
Beta particles (
) – electrons; negative charge
0
1 e
Gamma rays (
) – high energy form of electromagnetic radiation emitted by a radioactive element
0
0
Neutron Activation Analysis
The technique of bombarding specimens with neutrons and measuring the resulting gamma-ray radioactivity
Advantage – provides a non-destructive method for identifying and quantitating trace elements
It has been employed for characterizing the trace elements present in metals, drugs, paint, soil, gunpowder residues, and hair.
X-Ray Diffraction
Technique for identifying crystalline materials
As x-rays penetrate the crystal, a portion of the beam is reflected by each of the atomic planes. They interact with one another to form a series of light and dark bands known as a diffraction pattern
Every compound is known to produce its own unique diffraction pattern, thus giving analysts a means for
“fingerprinting” compounds
Drawback – not very sensitive and often fails to detect the presence of substances comprising less than 5% of a mixture
The Microscope
An optical instrument that uses a lens or combination of lenses to magnify and resolve the fine details of an object
Images
Virtual image
Can only be seen by looking through a lens and cannot be viewed directly
Real image
Can be viewed directly
Types of Microscopes
Magnifying glass
Magnification of 5-10 times
Single lens
Compound Light Microscope
Comparison Microscope
Stereoscopic Microscope
Polarizing Microscope
Microspectrophotometer
Scanning Electron Microscope (SEM)
Compound Light Microscope
Magnification up to 1500 times
Multiple lenses
Objective lens
lower lens of a microscope that is positioned directly over the specimen
Eyepiece lens
lens of a microscope into which the viewer looks, same as ocular lens
Mechanical system
base – support on which instrument rests arm – C-shaped/handle/support stage – horizontal plate upon which specimens are placed for study body tube – hollow tube on which objective and eyepiece are mounted at opposite ends coarse adjustment – focuses lenses fine focus – focuses but on smaller magnitude
Optical system
Illuminator
transmitted illumination – light that passes up from condenser and through specimen vertical or reflected illumination – illumination of a specimen from above
Condenser – collects light rays from illuminator and concentrates them onto specimen
Objective lens – lens closest to specimen
most microscopes are parfocal – when an image is focused with one objective in position, the other objective can be rotated into place and then the field will remain in focus
Eyepiece or ocular lens
monocular – one eyepiece binocular – two eyepieces
Optical system (cont.)
Magnifying power : power of objective lens x power of eyepiece lens
Numerical aperture
The ability of an objective lens to resolve details into separate images instead of one blurred image is directly proportional to the numerical aperture.
Ex. A lens with a NA of 1.30 can separate details that are twice as close as compared to lens with NA of 0.65.
Field of view
the area of the specimen that can be seen after it is magnified
as magnifying power increases, field of view decreases
Depth of focus
the thickness of a specimen entirely in focus under a microscope decreases as magnifying power increases
Comparison Microscope
Two compound microscopes combined into one unit
Uses a bridge incorporating a series of mirrors and lenses to join them
Very useful in forensic science when side-by-side comparisons are necessary
Vertical or reflected illumination
Used when comparing bullets, cartridges, other opaque objects
Transmitted illumination
Compare hairs or fibers
Stereoscopic Microscope
Power of 10x to 125x
Can present a 3-D image of object
Formation of right side up image
Very frequently used in crime lab
Wide field of view and great depth of focus
Used often for examination of paint, soil, gunpowder residues, marijuana, etc.
Polarizing Microscope
When a beam of light passes through certain types of substances, it emerges vibrating in only one plane.
Plane-polarized light: Light confined to a single plane of vibration
Polarizing Microscope (cont.)
Polarizer: Device that permits passage of light waves vibrating in only one plane
Second polarizing crystal – analyzer
Can modify stereo or compound microscopes so they can detect polarized light
Application – study materials that polarize light
ex. Birefringent substances
Microspectrophotometer
An instrument that links microscope to a spectrophotometer
Spectrophotometer an instrument used to measure and record the absorption spectrum of a chemical substance
Allows better comparison of substances
Scanning Electron Microscope (SEM)
Image is produced by aiming a beam of electrons onto the specimen and studying electron emissions on a closed TV circuit or computer
High magnification
High resolution
Scanning Electron Microscope
(SEM) (cont.)
High depth of focus
Used as tool for determining whether or not a suspect has recently fired a gun
X-ray analyzer