Mitochondria are the power station of the cell 5
advertisement

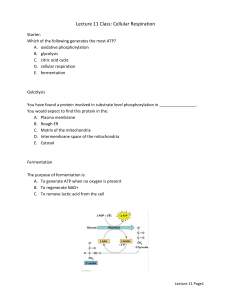
5 Mitochondria are the power station of the cell 1 5.1 Biological oxidation is preceded by a degradation of substrates to form bound hydrogen and CO2 2 • The total reaction of biological oxidation is equivalent to combustion with the difference that biological oxidation proceeds in a sequence of partial reactions, which allow the major part of the free energy to be utilized for synthesis of ATP. • The principle of biological oxidation was formulated in 1932 by the Nobel Prize winner Heinrich Wieland: 3 • First, hydrogen is removed from substrate XH2 and afterward oxidized to water. • Thus, during oxidation, carbohydrates [CH2O]n are first degraded by reaction with water to form CO2 and bound hydrogen [H], and the latter is then oxidized to water: 4 • In 1934 Otto Warburg (winner of the 1931 Nobel Prize in Medicine) showed that the transfer of hydrogen from substrates to the site of oxidation occurs as bound hydrogen in the form of NADH. • From results of studies with homogenates from pigeon muscle in 1937, Hans Krebs formulated the citrate cycle as a mechanism for substrate degradation, yielding the reducing equivalents for the reduction of oxygen via biological oxidation. In 1953 he was awarded the Nobel Prize in Medicine for this discovery. 5 5.2 Mitochondria are the sites of cell respiration 6 • Light microscopic studies of many different cells showed that they contain small granules, similar in appearance to bacteria. • At the beginning of the last century, the botanist C. Benda named these granules mitochondria, which means threadlike bodies. • For a long time, however, the function of these mitochondria remained unclear. 7 • As early as 1913, Otto Warburg realized that cell respiration involves the function of granular cell constituents. • He succeeded in isolating a protein from yeast that he termed “Atmungsferment” (respiratory ferment), which catalyzes the oxidation by oxygen. • He also showed that iron atoms are involved in this catalysis. 8 • In 1925 David Keilin from Cambridge (England) discovered the cytochromes and their participation in cell respiration. • Using a manual spectroscope, he identified the cytochromes-a, -a3, -b, and -c 9 • In 1928 Otto Warburg showed that his “Atmungsferment” contained cytochrome-a3. • A further milestone in the clarification of cell respiration was reached in 1937, when Hermann Kalckar observed that the formation of ATP in aerobic systems depends on the consumption of oxygen. • The interplay between cell respiration and ATP synthesis, termed oxidative phosphorylation, was now apparent. 10 • In 1948 Eugene Kennedy and Albert Lehninger showed that mitochondria contain the enzymes of the citrate cycle and oxidative phosphorylation. • These findings demonstrated the function of the mitochondria as the power station of the cell. 11 Figure 5.1 Scheme of mitochondrial energy metabolism. 12 5.3 Degradation of substrates for biological oxidation takes place in the matrix compartment 13 • Pyruvate, which is formed by the glycolytic catabolism of carbohydrates in the cytosol, is the starting compound for substrate degradation by the citrate cycle (Fig. 5.2). • Pyruvate is first oxidized to acetate (in the form of acetyl coenzyme A), which is then completely degraded to CO2 by the citrate cycle, yielding 10 reducing equivalents [H] to be oxidized by the respiratory chain to generate ATP. • Figure 5.3 shows the reactions of the citrate cycle. 14 Figure 5.2 Overall reaction of the oxidation of pyruvate by mitochondria. The acetate is formed as acetyl coenzyme A. [H] means bound hydrogen in the form of NADH and FADH2, respectively. 15 Figure 5.3 Scheme of the citrate cycle. The enzymes are localized in the mitochondrial matrix, with the exception of succinate dehydrogenase, which is located in the inner mitochondrial membrane. As a special feature, plant mitochondria contain NAD malic-enzyme in the mitochondrial matrix. Therefore plant mitochondria are able to oxidize malate via the citrate cycle also in the absence of pyruvate. Glutamate dehydrogenase enables mitochondria to oxidize glutamate. 16 Pyruvate is oxidized by a multienzyme complex • Pyruvate oxidation is catalyzed by the pyruvate dehydrogenase complex, a multienzyme complex located in the mitochondrial matrix. • It consists of three different catalytic subunits: pyruvate dehydrogenase, • dihydrolipoyl transacetylase, and • dihydrolipoyl dehydrogenase (Fig. 5.4). • The pyruvate dehydrogenase subunit contains thiamine pyrophosphate (TPP, Fig. 5.5A) as the prosthetic group. 17 • The reactive group of TPP is the thiazole ring. • Due to the presence of a positively charged Natom, the thiazole ring contains an acidic Catom. • After dissociation of a proton, a carbanion is formed, which is able to bind to the carbonyl group of the pyruvate. • The positively charged N-atom of the thiazole ring enhances the decarboxylation of the bound pyruvate to form hydroxethyl-TPP (Fig. 5.4). • The hydroxethyl group is now transferred to lipoic acid. 18 Figure 5.4 Oxidation of pyruvate by the pyruvate dehydrogenase complex, consisting of the subunits: - pyruvate dehydrogenase (with the prosthetic group Thiamine pyrophosphate), -Dihydrolipoyl Transacetylase (prosthetic group lipoic acid), - and dihydrolipoyl Dehydrogenase (prosthetic group FAD). 19 Figure 5.5 Reactionpartners of pyruvate oxidation: A. Thiamine pyrophosphate B. Lipoic amide, C. Coenzyme A. 20 Figure 5.6 Condensation of acetyl CoA with oxaloacetate to form citrate. 21 Figure 5.7 Isomerization of citrate to form isocitrate 22 Figure 5.8 Oxidation of isocitrate to form succinyl CoA. 23 Figure 5.9 Conversion of succinyl CoA to form oxaloacetate. 24 A loss of intermediates of the citrate cycle is replenished by anaplerotic reactions • citrate and α-ketoglutarate are withdrawn from the citrate cycle to synthesize the carbon skeletons of amino acids in the course of nitrate assimilation. • It is necessary, therefore, to replenish the loss of citrate cycle intermediates by anaplerotic reactions. 25 • Plant mitochondria are able to transport oxaloacetate into the chloroplasts via a specific translocator of the inner membrane. • Therefore, the citrate cycle can be replenished by the uptake of oxaloacetate, which has been formed by phosphoenolpyruvate carboxylase in the cytosol. • Oxaloacetate can also be delivered by oxidation of malate in the mitochondria. • Malate is stored in the vacuole and is an important substrate for mitochondrial respiration. 26 • A special feature of plant mitochondria is that malate is oxidized to pyruvate with the reduction of NAD and the release of CO2 via NAD-malic enzyme in the matrix. • Thus an interplay of malate dehydrogenase and NAD malic-enzyme allows citrate to be formed from malate without the operation of the complete citrate cycle. 27 • Another important substrate of mitochondrial oxidation is glutamate, which is one of the main products of nitrate assimilation. • besides sucrose, glutamate is the most highly concentrated organic compound in the cytosol of many plant cells. • Glutamate oxidation, accompanied by formation of NADH, is catalyzed by glutamate dehydrogenase located in the mitochondrial matrix. • This enzyme also reacts with NADP. NADPglutamate dehydrogenase activity is also found in plastids, although its function there is not clear. 28 • Glycine is the main substrate of respiration in the mitochondria from mesophyll cells of illuminated leaves. 29 5.4 The mitochondrial respiratory chain shares common features with the photosynthetic electron transport chain 30 First cyanobacteria.. • The photosynthesis of cyanobacteria led to the accumulation of oxygen in the early atmosphere and • Thus formed the basis for the oxidative metabolism of mitochondria. 31 • Cyanobacteria contain a photosynthetic electron transport chain that consists of three modules (complexes), namely, 1. photosystem II, 2. cyt-b6/f complex, 3. photosystem I These complexes are located in the inner membrane of cyanobacteria, where, however, there are also the enzymes of the respiratory electron transport chain. 32 33 Figure 5.12 : Scheme of photosynthetic and oxidative electron transport in cyanobacteria. In both electron transport chains the cytochrome-b6/f complex functions as the central complex. Figure 5.13 Scheme of mitochondrial electron transport. The respiratory chain consists of three complexes; the central cytb/c1 complex corresponds to the cyt-b6/f complex of cyanobacteria and chloroplasts. 34 Figure 5.15 Scheme of the location of the respiratory chain complexes I, III, and IV in the mitochondrial inner membrane. 35 5.5 Plant mitochondria have special metabolic functions 36 • In plant cells performing photosynthesis, the role of the mitochondria as a supplier of energy is not restricted to the dark phase; • The mitochondria provide the cytosol with ATP also during photosynthesis. • In addition, the mitochondrial matrix contains enzymes for the oxidation of glycine to serine, an important step in the photorespiratory pathway. 37 • The NADH generated from glycine oxidation is the main fuel for mitochondrial ATP generation during photosynthesis. • Another important role of plant mitochondria is the conversion of oxaloacetate and pyruvate to form citrate, a precursor for the synthesis of α-ketoglutarate. • This pathway is important for providing the carbon skeletons for amino acid synthesis during nitrate assimilation. 38