Calculating properties of non
advertisement

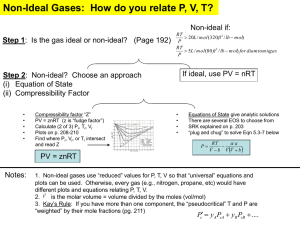
Non-tabular approaches to calculating properties of real gases The critical state • At the critical state (Tc, Pc), properties of saturated liquid and saturated vapor are identical • if a gas can be liquefied at constant T by application of pressure, T·Tc. • if a gas can be liquefied at constant P by reduction of T, then P·Pc. • the vapor phase is indistinguishable from liquid phase Properties of the critical isotherm • The SLL and SVL intersect on a P-v diagram to form a maxima at the critical point. •On a P-v diagram, the critical isotherm has a horizontal point of inflexion. – – P 0 v Tc 2P 2 0 v Tc Departures from ideal gas and the compressibility factor Pv 1 RT • For an ideal gas • One way of quantifying departure from ideal gas behavior to evaluate the “compressibility factor” (Z) for a true gas: Pv v Z RT videal • Both Z<1 and Z>1 is possible for true gases The critical state and ideal gas behavior • At the critical state, the gas is about to liquefy, and has a small specific volume. videal vtable 100% vtable is very large Z factor can depart significantly from 1. Whether a gas follows ideal gas is closely related to how far its state (P,T) departs from the critical state (Pc, ,Tc). Critical properties of a few engineering fluids • Water/steam (power plants): – CP: 374o C, 22 MPa – BP: 100o C, 100 kPa (1 atm) • R134a or 1,1,1,2-Tetrafluoroethane (refrigerant): – CP: 101o C, 4 MPa – BP: -26o C, 100 kPa (1 atm) • Nitrogen/air (everyday, cryogenics): – CP: -147o C, 3.4 MPa – BP: -196o C, 100 kPa (1 atm) Principle of corresponding states (van der Waal, 1880) • • • • Reduced temperature: Tr=T/Tcr Reduced pressure: Pr=P/Pcr Compressibility factor: Principle of corresponding states: All fluids when compared at the same Tr and Pr have the same Z and all deviate from the ideal gas behavior to about the same degree. Generalized compressibility chart 1949 Fits experimental data for various gases Use of pseudo-reduced specific volume to calculate p(v,T), T(v,p) using GCC Z Nelson-Obert generalized compressibility chart 1954 Based on curvefitting experimental data Equations of state Some desirable characteristics of equations of state • Adjustments to ideal gas behavior shoujd have a molecular basis (consistency with kinetic theory and statistical mechanics). • Pressure increase leads to compression at P constant temperature v 0 • Critical isotherm has a horizontal point of P P 0, inflection: v v 0, • Compressibility factor (esp. at critical state consistent with experiments on real gases.) T 2 2 Tc Tc Some equation of states Often • Two-parameter equations of state based on theory • Virial equation of states Z=1+A(T)/v+B(T)/v2+…. (coefficients can be determined from statistical mechanics) • Multi-parameter equations of state with empirically determined coefficients: – Beattie-Bridgeman – Benedict-Webb-Rubin Equation of State Two-parameter equations of states • Examples: – Van der waals – Dieterici – Redlich Kwong P RT / (v b) a / v 2 P RT a exp vb RTv P RT a v b T v (v b ) • Parameters (a, b) can be evaluated from critical point data using Pv 0, vP 0, • Van der Waals: 2 2 Tc Tc 27 R 2Tc2 RTc a ; b ; Zc 0.375 64 Pc 8 pc Critical compressibility of real gases First law in differential form, thermodynamic definition of specific heats