投影片 1
advertisement

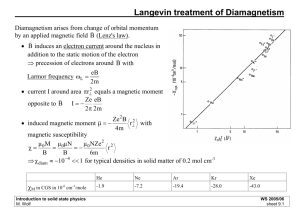
11. Diamagnetism and Paramagnetism • • • • • • • • • • • • • • Ref: Langevin Diamagnetism Equation Quantum Theory of Diamagnetism of Mononuclear Systems Paramagnetism Quantum Theory of Paramagnetism Rare Earth Ions Hund Rules Iron Group Ions Crystal Field Splitting Quenching of The Orbital Angular Momentum Spectroscopic Splitting Factor Van Vieck Temperature-Independent Paramagnetism Cooling by Isentropic Demagnetization Nuclear Demagnetization Paramagnetic Susceptibility of Conduction Electrons D.Wagner, “Introduction to the Theory of Magnetism”, Pergamon Press (72) Bohr-van Leeuwen Theorem M = γ L = 0 according to classical statistics. → magnetism obeys quantum statistics. Main contribution for free atoms: • spins of electrons • orbital angular momenta of electrons • Induced orbital moments paramagnetism Electronic structure Moment H: 1s MS He: 1s2 M=0 unfilled shell M0 All filled shells M=0 diamagnetism Magnetization M magnetic moment per unit volume Magnetic subsceptibility per unit volume χ M = molar subsceptibility σ = specific subsceptibility M H In vacuum, H = B. nuclear moments ~ 10−3 electronic moments Larmor Precession 1 3 J x A d x c x x Magnetic (dipole) moment: For a current loop: 1 d 3 x x J x 2c m J d 3x I d l For a charge moving in a loop: m m 1 2c A x I dl J x q v x xq q 1 q 3 d x x q v x x x v L q q 2c 2c 2mc Classical gyromagnetic ratio Torque on m in magnetic field: q 2mc Γ Lorentz force: dv q vB dt c I Area c ( charge at xq ) Caution: we’ll set L to L in the quantum version L B e 2mc dL mB LB dt → L precesses about B with the Larmor frequency m mx r3 → cyclotron frequency qB 2mc qB c 2L mc L B Langevin Diamagnetism Equation Diamagnetism ~ Lenz’s law: induced current opposes flux changes. L Larmor theorem: weak B on e in atom → precession with freq Larmor precession of Z e’s: L Z e2 B I Ze 2 4 m c 2 x2 y 2 For N atoms per unit volume: 1 I c r 2 x2 y 2 z 2 1 eB C 2 2mc r2 → N N Z e2 2 r B 6 m c2 χ<0 2 Z e2 B 4 m c2 2 3 2 2 Langevin diamagnetism same as QM result Good for inert gases and dielectric solids experiment Failure: conduction electrons (Landau diamagnetism & dHvA effect) Quantum Theory of Diamagnetism of Mononuclear Systems Quantum version of Langevin diamagnetism ie e2 H A2 A A 2 2mc 2mc Perturbation Hamiltonian [see App (G18) ]: Uniform B B zˆ → A 1 B y , x, 0 2 → A 0 A 1 By x 2 x y i eB e2 B 2 2 2 eB e2 B 2 2 2 H x y x y Lz x y 2 4 m c y x 8 m c 2mc 8 m c2 The Lz term gives rise to paramagnetism. 1st order contribution from 2nd term: e2 r 2 E B B 6 m c2 e2 B 2 2 E 8 m c2 e2 B 2 2 r 12 m c 2 same as classical result Paramagnetism Paramagnetism: χ > 0 Occurrence of electronic paramagnetism: • Atoms, molecules, & lattice defects with odd number of electrons ( S 0 ). E.g., Free sodium atoms, gaseous NO, F centers in alkali halides, organic free radicals such as C(C6H5)3. • Free atoms & ions with partly filled inner shell (free or in solid), E.g., Transition elements, ions isoelectronic with transition elements, rare earth & actinide elements such as Mn2+, Gd3+, U4+. • A few compounds with even number of electrons. E.g., O2, organic biradicals. • Metals Quantum Theory of Paramagnetism μ Magnetic moment of free atom or ion: γ = gyromagnetic ratio. g = g factor. g B For electrons g = 2.0023 For free atoms, g 1 U μ B mJ g B B N eB B N e e B μB = Bohr magneton. B J LS Caution: J here is dimensionless. e ~ spin magnetic moment of free electron 2mc J J 1 S S 1 L L 1 2 J J 1 mJ J , J 1, For a free electron, L = 0, S = ½ , g = 2, → mJ = ½ , U = μB B. N e B N e B e B J g B J , J 1, J Anomalous Zeeman effect x B k BT N e x x N e e x N ex x N e e x e x e x M N N N x N tanh x e e x High T ( x << 1 ): N 2 B M N x kB T Curie-Brillouin law: M N g J B BJ x x g J B B kB T Brillouin function: BJ x 2 J 1 x 1 2J 1 x ctnh ctnh 2J 2 J 2 J 2 J M N g J B BJ x BJ x x g J B B kB T 2 J 1 x 1 2J 1 x ctnh ctnh 2J 2J 2J 2J High T ( x << 1 ): 1 x x3 ctnh x x 3 45 2 2 N p 2 B2 C M N J J 1 g B 3 k T B 3 k BT T B pg N J J 1 Curie law = effective number of Bohr magnetons Gd (C2H3SO4) 9H2O Rare Earth Ions Lanthanide contraction ri = 1.11A ri = 0.94A 4f radius ~ 0.3A Perturbation from higher states significant because splitting between L-S multiplets ~ kB T Hund’s Rules For filled shells, spin orbit couplings do not change order of levels. Hund’s rule ( L-S coupling scheme ): Outer shell electrons of an atom in its ground state should assume 1.Maximum value of S allowed by exclusion principle. 2.Maximum value of L compatible with (1). 3.J = | L−S | for less than half-filled shells. J = L + S for more than half-filled shells. Causes: 1. Parallel spins have lower Coulomb energy. 2. e’s meet less frequently if orbiting in same direction (parallel Ls). 3. Spin orbit coupling lowers energy for LS < 0. Mn2+: 3d 5 (1) → S = 5/2 Ce3+: 4f1 L = 3, S = ½ Pr3+: 4f2 (1) → S = 1 exclusion principle → L = 2+1+0−1−2 = 0 (3) → J = | 3− ½ | = 5/2 (2) → L = 3+2 = 5 2 F5/2 (3) → J = | 5− 1 | = 4 3 H4 Iron Group Ions L=0 Crystal Field Splitting Rare earth group: 4f shell lies within 5s & 5p shells → behaves like in free atom. Iron group: 3d shell is outer shell → subject to crystal field (E from neighbors). → L-S coupling broken-up; J not good quantum number. Degenerate 2L+1 levels splitted ; their contribution to moment diminished. Quenching of the Orbital Angular Momentum Atom in non-radial potential → Lz not conserved. If Lz = 0, L is quenched. μ B L 2S L is quenched → μ is quenched L = 1 electron in crystal field of orthorhombic symmetry ( α = β = γ = 90, a b c ): e A x2 B y 2 C z 2 2 0 U j xj f r Consider wave functions: For i j, the integral i.e., Ui e U j d rU 3 Similarly * i Uj → e Ax2 By 2 A B z 2 L 2 U j L L 1 U j 2 U j → is odd in xi & xj , and hence vanishes. i j Ui e Ui U x e U x d 3r f r where A B C 0 I1 d 3r f r Uy e Uy 2 2 A x 4 B x 2 y 2 A B x 2 z 2 A I1 I 2 x 4j B I1 I 2 I 2 d 3r f r 2 xi2 x 2j U z e U z A B I1 I 2 Uj are eigenstates for the atom in crystal field. Orbital moments are zero since U j Lz U j 0 Quenching For lattice with cubic symmetry, e A x2 y 2 z 2 2 0 A0 there’s no quadratic terms in e φ . → Ground state remains triply degenerate. Jahn-Teller effect: energy of ion is lowered by spontaneous lattice distortion. E.g., Mn3+ & Cu2+ or holes in alkali & siver halides. Spectroscopic Splitting Factor λ = 0 or H = 0 → Uj degenerate wrt Sz. In which case, let A, B be such that ψ0 = x f(r) α is the ground state, where α (spin up) and β (spin down) are Pauli spinors. 1st order perturbation due to λ LS turns ψ0 into U x i U y Uz 21 2 2 where 1 y x 2 z x α | β = 0 → term Uz β ~ O(λ2) in any expectation values. It can be dropped in any 1st approx. Lz Thus 1 z B Lz 2Sz 1 B 1 Energy difference between Ux α and Ux β in field B : → g 2 1 1 E g B H 2 1 B H 1 Van Vleck Temperature-Independent Paramagnetism Consider atomic or molecular system with no magnetic moment in the ground state , i.e., 0 z 0 s z s 0 In a weak field μz B << Δ = εs – ε0 , 0 0 0 B s 0 z 0 s z s s z 0 0 z s z a) Δ << kB T N0 N s M 2B N 0 2 B s z 0 s 2 B s z 0 B 0 0 z s 2 2 b) Δ >> kB T N 2 k BT N0 N s N 2 s z 0 s z 0 s s s 2 N 2 kB T 1 kB T 2B s z 0 2 2N s z 0 2 M Curie’s law N van Vleck paramagnetism Cooling by Isentropic Demagnetization Was 1st method used to achieve T < 1K. Lowest limit ~ 10–3 K . Mechanism: for a paramagnetic system at fixed T, Δ S < 0 as H increases. i.e., H aligns μ and makes system more ordered. → Removing H isentropically (Δ S = 0) lowers T. Lattice entropy can seeps in during demagnetization Magnetic cooling is not cyclic. Isothermal magnetization Isoentropic demagnetization T2 Spin entropy if all states are accessible: S k B ln 2S 1 N N kB ln 2S 1 S is lowered in B field since lower energy states are more accessible. Population of magnetic sublevels is function of μB/kBT, or B/T. ΔS=0→ B B T1 T2 or T2 T1 B B BΔ = internal random field Nuclear Demagnetization T2 T1 m p ~ 1836 me g p ~ 5.58 B B 5.58 3 → p ~ 2 1836 e ~ 1.52 10 B gn ~ 3.83 T2 = T1 ( 3.1 / B ) → T2 of nuclear paramagnetic cooling ~ 10–2 that of electronic paramagnetic cooling. B = 50 kG, T1 = 0.01K, → B kB p B k B T1 0.5 6.72 105 G / K ΔS on magnetization is over 10% Smax. → phonon Δ S negligble. Cu: T1 = 0.012K BΔ =3.1 G 102 100G T2 0.01K 2 107 K 50kG Paramagnetic Susceptibility of Conduction Electrons Classical free electrons: N B2 B M k BT ~ Curie paramagnetism Experiments on normal non-ferromagnetic metals : M independent of T Pauli’s resolution: Electrons in Fermi sea cannot flip over due to exclusion principle. Only fraction T/TF near Fermi level can flip. N B2 B T N B2 B M kBT TF k B TF Pauli paramagnetism at T = 0 K T=0 1 F 1 F 1 N d D B d D B D F 2 B 2 B 2 parallel moment 1 F 1 F 1 N d D B d D B D F 2 B 2 B 2 M Pauli N N B D F 2 Landau diamagnetism: M Landau 3N 2 B 2k BTF N2 B 2k BTF → χ is higher in transition metals due to higher DOS. anti-parallel moment χ > 0 , Pauli paramagnetism M M Pauli M Landau N2 B 2k BTF Prob. 5 &6