Syllabus and Schedule - Emporia State University
advertisement

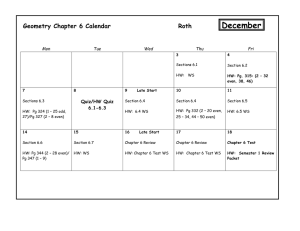
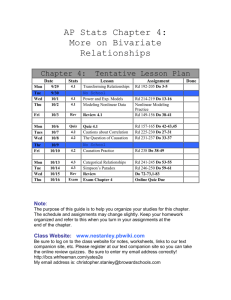
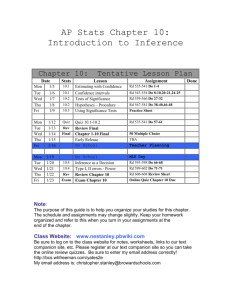
CH 114 – Introduction to Physical Inorganic Chemistry I Fall 2009; MWF 8-8:55 AM, C225 BAC (lab VLCS 2019) Section 01 – Lab 1-4:50 PM Mon Prof. Diane L. Nutbrown Office Hours 2039 VLCS M 9 - 11 AM nutbrown@juniata.edu W 11 AM – Noon TBA x3718 1 – 2 PM Section 02 – Lab 1-4:50 PM Wed Dr. Peter Baran 2035 VLCS baran@juniata.edu x3560 - - - or by appointment - - Problem-solving session R 4-5:30 PM, VLCS 1022 Course Overview and Goals This course offers environmental science, geology, and engineering POEs a foundation in general chemistry. As such, the concepts covered throughout the class parallel content from a stereotypical “freshman chemistry” course at the college level. These concepts, however, will be discussed within the context of environmental science, geology, and engineering as much as possible to reinforce their relevance and application. In addition to learning chemistry concepts, this course is designed to advance students’ skills in the areas of problem-solving, communication, and teamwork. Activities and assignments will move you and your group members from completing simple exercises to solving complex problems. Critical thinking is a primary outcome of a liberal arts education; thinking like a scientist is thinking critically! CH 114 will give you ample opportunities to improve your communication skills through oral discussion, written assessments, and lab reports, as described below. To strengthen your ability to work in a team, collaborative learning will be emphasized throughout the course. Required Text Bell, J.A. Chemistry: a project of the American Chemical Society; W.H. Freeman: New York, 2005. Technology E-mail communication will be sent to your juniata.edu account only, and correspondence sent to course instructors/TAs should contain “Chem 114” in the subject line. Please take advantage of this tool. The CH 114 Moodle course primarily operates as a repository of course materials. Laboratory procedures, learning objectives, answer keys, and practice exams will be posted, as well as the announced homework problems and reading assignments from class. You will also submit lab reports through this medium. Be sure to register and check the course page regularly. Clickers will be used in class to check how well you and your peers understand the material, or simply to gather data about an activity. Your answer will always be anonymous to the rest of the class and your honest response helps inform the pace of the lesson. You are expected to participate with your clicker during each lesson, as described below in the active learning section. Active Learning In order to learn chemistry (or any subject/skill), you need to actively engage in the material and spend time on task. Some students lament that they put in lots of effort, yet never see the results. I would like to address that complaint by assigning credit to your active learning. Class participation – You will automatically earn a 9/10 for each day you come to class and submit answers to 100% of the clicker questions posed. You can increase to a 10/10 by initiating questions or contributing to discussions. You can also lose points by failing to submit answers, disrupting the learning environment, or simply being absent. Class participation represents 60% of the Active Learning grade Homework Journal – All homework problems must be kept in a sewn-bound journal (no spiralbound, no three-ring binder, no loose leaf). Homework problems will be assigned at each class session. You are encouraged to keep current with the problems and to work on them with your group members. Homework journals will be collected at random times and graded on a lowresolution scale based on effort and correctness. The total points available will depend on the number of problems graded in each set. It will be your responsibility to compare your answers to the solutions posted on the class Moodle site and to come talk with Prof. Nutbrown if you do not understand how to arrive at a correct answer for each assigned problem. Suggested problems should also be completed in your homework journal, but worked from the back of the notebook forward so as to not interfere with the problem sets in the front. Never come to class, office hours or problem-solving sessions without your homework journal. The Homework Journal represents 40% of the Active Learning grade Office Hours/Problem-solving sessions – Demonstrate that you’re working on the material outside of class and take advantage of time with the professor. Your attendance at either office hours or the weekly problem-solving session will not be required, but may be strongly encouraged if you have indicated a weak high school background in chemistry or if you perform poorly on the first few quizzes. All students are welcome, however, regardless of skill level. Attendance will bolster your active learning grade, but absence will have no effect. Bonus opportunities – There may be occasional bonus opportunities throughout the course. The points earned will only benefit the active learning grade. Choosing to not participate will leave your grade unaltered. Quizzes Learning objectives will be provided in advance for each in-class quiz. The quizzes will be given on Fridays beginning August 28 as noted on the attached schedule. Quizzes are designed to quickly assess your skill in applying the tools taught in class and will take no more than 15 minutes to complete. Each quiz is scored out of 10 points; your lowest quiz score will be dropped. Exams Three 75-point out-of-class exams will be administered after the completion of most chapters. Exam objectives and a practice exam will be posted in the Moodle course to help you prepare for each test. The 125-point final exam will contain questions representing all content areas discussed during the semester. Although exams 2 and 3 are not explicitly cumulative, these tests are also likely to assess knowledge and skills from the previous units, because concepts will build upon one another. Exams are tentatively scheduled for the following dates/times: 1 = Thurs. 10/8/09, 7-10 PM 2 = Thurs. 11/5/09, 7-10 PM 3 = Mon. 11/23/09, 1-4 PM These blocks of time allow you to take the hour exam in 1.5 h individually and then re-take the exam with your group. Your total exam score will be calculated as (0.75*indiv.) + (0.25*group). Laboratory Chemistry is an experimental science, which makes the laboratory portion of the course an essential component for learning. Although the lab should be a chance to explore and have fun, you are expected to exercise common sense and professional behavior. Safe lab practices should be followed, including: wearing appropriate clothing and personal protective equipment, disposing of chemicals properly, and maintaining a clean workspace. Attendance policy Lab attendance is mandatory. If you are involved in college athletics and have a conflict with any of the sessions, it is your responsibility to alert to alert the instructor(s) immediately so that arrangements may be made to accommodate the missed work. Notebook A hardback, sewn notebook is required for use as a laboratory notebook. It is your choice whether the paper is lined/ruled or graph/quad. Your laboratory notebook will be checked for preparation, completeness, and overall organization during each session. Hence, your notebook should contain a title, experimental objective, background/calculations, outlined procedure, and pre-organized data tables (if appropriate) when you arrive to lab. As you experiment, take notes in real time...and take them in your notebook, not on scraps of paper! Maintaining an accurate, up-to-date record of your actions in the lab can sometimes be a challenge, so it is a good idea to begin developing excellent habits from the beginning. Be sure to reserve the first few pages at the front of the notebook for a table of contents, number all pages, and only write on one side of the page to leave room for feedback from instructors. Reports Lab reports will be completed and submitted as a group. Details about lab reports will be given throughout the semester. Lab quizzes/assignments Most labs will involve a short, graded 5-point quiz at the beginning of the period that addresses concepts or techniques relevant to the investigation. Rather than begin by writing a complete lab report, you will start by composing segments. These segments will be required as assignments during the first few weeks of lab, and other similar activities may be given in later weeks. Grades Total grade = (0.10*Active Learning) + (0.10*Quizzes) + (0.20*Lab) + (0.60*Exams) Letter % A 100-92 A92-90 B+ 90-87 B 87-82 B82-80 C+ 80-77 C 77-72 C72-70 D 70-55 F 55> Learning Support and Special Concerns Any student with a documented disability who needs to arrange reasonable accommodations to enable their success in this course is encouraged to contact the Office of Academic Support Services (x3161) at the beginning of the semester. You are also encouraged to schedule an appointment to discuss your learning with Prof. Nutbrown. If you are unable to attend class because of an unavoidable schedule conflict, for example, a religious observance, an athletic activity, or a family obligation, contact Prof. Nutbrown to make up any missed work (e.g. in-class quiz). If circumstances arise unexpectedly that preclude you from taking an exam, please contact Prof. Nutbrown before the scheduled exam time. It is understood that, in an emergency situation, you may not be able to do this in a timely way. Week 1 2 3 4 5 6 7 8 9 10 11 Day Mon Wed Fri Mon Wed Fri Mon Wed Fri Mon Wed Fri Mon Wed Fri Mon Wed Fri Mon Wed Fri Mon Wed Fri Mon Wed Fri Mon Wed Fri Mon Wed Fri Date 8/24 8/26 8/28 8/31 9/2 9/4 9/7 9/9 9/11 9/14 9/16 9/18 9/21 9/23 9/25 9/28 9/30 10/2 10/5 10/7 10/9 10/12 10/14 10/16 10/19 10/21 10/23 10/26 10/28 10/30 11/2 11/4 11/6 Topic welcome, course overview, states of matter, phase changes Lab # Quiz # safety & pre-assessments 1 matter, density, nuclear model of the atom Lab 1 2 quantum theory Lab 2 3 periodic trends Lab 3 4 Lewis structures Lab 4 5 molecular representations cont.: resonance, dipoles/polarity Lab 5 6 VSEPR theory, intermolecular forces intermolecular forces Lab 6 Exam 1 Lab 7 7 NO CLASS – FALL BREAK NO LAB ionic compounds – nomenclature and conductivity ionic compounds – reactions and solubility rules 8 Lab 8 9 concentration, mole/mass/volume conversions Lab 9 Exam 2 Week 12 13 14 15 16 Day Mon Wed Fri Mon Wed Fri Mon Wed Fri Mon Wed Fri Mon Date 11/9 11/11 11/13 11/16 11/18 11/20 11/23 11/25 11/27 11/30 12/2 12/4 12/7 Topic Lab # Quiz # Lab 10 10 solution chemistry, cont. Lab 11 classifying chemical reactions NO LAB 11 Exam 3 NO CLASS – THANKSGIVING BREAK Lab 12 reaction stoichiometry, limiting reagent 12