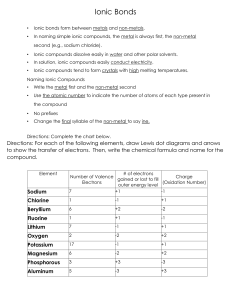
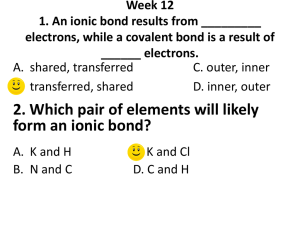
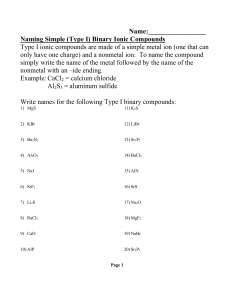
Ionic Bonding Test Review In an ionic bond, electrons are between
advertisement

Ionic Bonding Test Review 1. In an ionic bond, electrons are ____________________ between atoms. 2. What are ions? 3. What is the difference between cations and anions? 4. What types of elements form an ionic bond? 5. What is the overall charge of a compound? 6. List properties of ionic compounds 7. For each of the following elements, identify if it will gain or lose electrons, and the charge of the ion it will form: a. Potassium: d. Oxygen: b. Phoshporus: e. Magnesium: c. Aluminum: f. Bromine: 8. Draw the Lewis Dot structure (and electron transfer) for the following sets of elements: d. Calcium & Fluorine a. Sodium & Nitrogen b. Lithium & Chlorine e. Beryllium & Phosphorus c. Magnesium & Sulfur 9. Name the following Ionic compounds (Pay attention to if it needs a roman numeral): a. Na2S ___________________________________________ b. Ca3N2 ___________________________________________ c. Pb3N2 ___________________________________________ d. Cu2O ___________________________________________ e. SnS2 ___________________________________________ f. CaCO3 ___________________________________________ g. NH4F ___________________________________________ h. Cu(NO2)2 ___________________________________________ i. NiPO4 ___________________________________________ j. (NH4)2SO3 ___________________________________________ 10. a. b. c. d. e. Write the chemical formula for the following compounds: Calcium bromide _________________ f. Ammonium bromide_________________ Aluminum oxide _________________ g. Calcium hydroxide _________________ Platinum (II) sulfide _________________ h. Ammonium nitrate _________________ Iron (II) chloride _________________ i. Tin (IV) sulfate _________________ Copper (I) sulfide _________________ j. Cobalt (III) chromate_________________