Preventing Hospital-Acquired Infections
advertisement

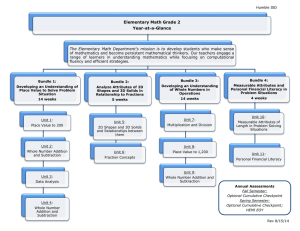
Preventing Central Line Associated Bloodstream Infections (CLABSIs) What Clinical Staff Should Know Prepared by Ann Bailey RNC-NIC, BSN, MBA, CIC Joanne Dixon MN, RN, CIC Gwen Irwin, RN, CRNI Judy Smith, RN, BSN, CRNI December 18, 2009 Objectives Upon completion of this module, the learner will be able to: • Summarize the Joint Commission 2010 National Patient Safety Goal 07.04.01 related to Central Line Associated Bloodstream Infections (CLABSIs), effective 01/01/10 • Includes using “Bundle” with respect to preventing CLABSIs • Define “Bundle” • Name 2 ways patients get CLABSIs • List 4 evidence-based practices that have been shown to help prevent CLABSIs The Joint Commission 2010 National Patient Safety Goal (NPSG) • NPSG 07.04.01 focuses on the prevention of CLABSIs. • All those who manage central lines MUST have education about the importance of preventing CLABSIs. • Includes staff, doctors, APNs or other licensed providers • Patients and families MUST be educated about CLABSI prevention before any central line insertion. • CLABSI surveillance will be hospital wide, not targeted to ICUs. • For adults, NO femoral catheters, unless other sites aren’t available. Patient and Family Education Before Central Line Insertion • FAQ Catheter Associated Bloodstream Infections from Joint Commission covers: • Providers doing hand hygiene • Steps for maximum barrier CVL insertion • Clean hands before using CVL • Clean connectors with antiseptic solution before using CVL • Decide every day if CVL is needed. • Ask providers to clean hands if patient doesn’t see them. • Tell nurse if dressing comes off or wet or dirty. • And more The Joint Commission 2010 National Patient Safety Goal (NPSG) • NPSG 07.04.01 focuses on the prevention of CLABSIs. • Also includes the CVL insertion bundle. • We have had in place for almost 5 years. • Also includes part of the CVL maintenance bundle. • We have had in place for about 2 years. What is a Bundle? • A grouping of evidence-based best practices that individually improve care, but when applied together result in substantially greater improvement. • Science behind the bundle elements is well established – the standard of care. • Bundle element compliance can be measured as “ yes/no.” • “All or none” approach. The CVL Insertion Bundle 1. 2. 3. 4. Hand hygiene immediately prior to insertion -wash hands or -use alcohol-based hand gel/foam Maximal barrier precautions -full body sterile drape -clinician and assistant wear cap, mask, sterile gown, gloves -persons within 6 feet wear hat and mask Skin antisepsis with chlorhexidine 2% / 70% isopropyl alcohol. Subclavian site considered 1st choice; avoid IJ & femoral. Exceptions: Should be rare for adults - Hemodialysis catheters - When high risk for pneumothorax - When high risk for noncompressible hematoma The CVL Maintenance Bundle 1. Perform good hand hygiene, prior to handling line -Hand washing or -Use alcohol-based hand gel/foam 2. Assess dressing/site with routine assessment 3. Scrub connector vigorously with alcohol x 15 seconds -Allow to dry before accessing 4. Assess line patency for brisk return and easy flushing 5. Assess to determine if patient meets criteria for line necessity Why Prevent CLABSIs? • Nationally and annually: • 80,000 central line associated bloodstream infections occur in ICUs • 250,000 hospital-wide, including ICUs • Seton Family of Hospitals • The majority of CLABSIs occur outside of the critical care units • Check your unit’s CLABSIs with your infection preventionist, if you are interested in more information • Increases the patient’s risk of death significantly • CLABSIs lead to longer length of stay (LOS) • National estimates show the cost of a BSI can be as high as $25,000 per episode (MMWR, August 9, 2002 Vol. 51, No. RR-10) How do CLABSIs happen? • Introduction of pathogens into the bloodstream from the skin around insertion site • Introduction of pathogens into the bloodstream from the hub or connector of the catheter. • Most frequent cause nationally • Also true at Seton Factors That Increase Risk of BSIs • CVLs in areas that have increased colonization of organisms • such as the internal jugular or femoral sites • Multiple lumens: More manipulation and contamination. (MMWR, August 9, 2002 Vol. 51, No. RR-10) • Use of stopcocks (MMWR, August 9, 2002 Vol. 51, No. RR-10) • Contamination of IV tubing or connectors (caps) • Longer dwell time of CVC Factors That Lower Risk of BSIs • Select subclavian site over internal jugular or femoral sites, if PICC not used • Perform hand hygiene • Use maximum barrier precautions • Skin prep with chlorhexidine rather than povidone-iodine • Skin prep on clean skin • Maintain patency of all lumens • Free of sluggishness or occlusion; brisk blood return • Remove line when no longer necessary CVL Insertion Bundle Component: CVL Site Choices Femoral Vein Last choice Subclavian Vein First Choice Internal Jugular Second choice Hand Hygiene – The Most Important Way to Prevent Any Infection Alcohol-based hand gel/foam - apply product to palm of one hand and rub hands together, covering all surfaces of hands and fingers until hands are dry Handwashing - 10-15 seconds of soap and friction, rinse, dry and turn off faucet with clean paper towel CVL Insertion Bundle Component: Maximum Sterile Barrier Precautions Sterile gown Hat and mask Sterile gloves Persons within 6 feet also wear hat and mask CVL Insertion Bundle Component: Chloraprep® • Gross debris or dirt should be removed • with an alcohol pad, prior to using the skin prep. • by washing with soap and water, prior to using the skin prep. • Clean with friction for minimum of 30 seconds. • Allow Chloraprep® to completely dry, before procedure for best results. • DO NOT REMOVE Chloraprep® after the procedure is completed. Exception: neonates <2 months. CVL Maintenance Bundle Component: Assess line patency for brisk return and easy flushing Research studies indicate a direct correlation with occlusions, fibrin sheaths, and risk of CLABSIs No blood return? Flushes easily? • Probable fibrin sheath or fibrin tail • Treat as soon as possible • Treat with Alteplase per declotting protocol Infusing around sheath Fibrin sheath Catheter Attempting to withdraw blood CVL Maintenance Bundle Component: Daily Review for Line Necessity Remove when No Longer Indicated Indications for a CVL • Hemodynamic monitoring • Administration of certain medications that require central administration, e.g. vasopressors, chemotherapy, TPN • Long term IV therapy, e.g. antibiotics or inotropes • Plasmapheresis, apheresis, hemodialysis, or continuous renal replacement therapy • Poor peripheral venous access, when IV treatment is still needed CVL Maintenance Bundle Component: Scrub the Hub with Alcohol for 15 seconds, prior to accessing • Vigorous scrubbing is necessary to remove pathogens Research shows that 5 seconds is not enough. • • • 67% of pathogens are still transferred. Research shows that 15 seconds with friction is 100% effective in disinfection. If this step is skipped, the patient is inoculated with the organisms of his surroundings. CVL Maintenance Bundle Component: Assess dressing/site with routine assessment Keep dressing clean dry and intact • Loose and wet dressings are sites of potential infection. • CHANGE THEM! • Cover the site dressing and the connectors during showers. Aquaguard is available: 7”x7” Lawson number 080204 Potential ways of contamination • The top of the medication vial is not sterile. • The top is a “dust cover.” • Clean vigorously with alcohol before accessing the vial with the blunt fill needle. Disconnecting tubing Sterile end cap in place Not recommended by manufacturer. Off-label use. How do you know if the tubing tip is still sterile? Indicates tip sterility maintained Some Prefilled Saline Syringes Are for Flushing ONLY • The saline flush syringes in the clear cellophane package is ONLY for flushing • According to the manufacturer, DO NOT use for medication dilution. • The inside of the barrel & the fluid pathway is all that is sterile on these syringes. • When you push out saline, the outer side of the plunger contaminates the inside of the barrel. • Then, when you draw back into the syringe, you are pulling the plunger over areas that were just contaminated. • If you do this, you could be pushing pathogens into the patients’ bloodstreams. • The saline flush syringes in the sterile peel pack may be used for medication dilution. Your Role • Follow the bundle components specific to your role in the patient’s care • Provide appropriate/indicated patient teaching regarding these bundle component and other recommended practices • Document patient education related to the goal of CLABSI prevention • Patient education materials related to CLABSI prevention can be found on the Intranet: • • • • http://intranet.seton.org/polandproc/infectcontrol/docs/clabsi.pdf http://intranet.seton.org/polandproc/infectcontrol/docs/clabsi_largertext.pdf http://intranet.seton.org/polandproc/infectcontrol/docs/clabsi_spanish.pdf http://intranet.seton.org/polandproc/infectcontrol/docs/clabsi_span_lg_txt.pdf • Remind peers of the importance of following the bundle components and other recommended practices if they are observed to be non-compliant Policies Central line insertion and dressing policies: Caring for Central Venous Catheters (CVC), (adult patients) Caring for Central Venous Catheters (CVC), e.g. Broviac, Hickman, Groshong, Hohn, Peripherally Inserted Central Catheter (PICC) (pediatric patients) Caring for Peripherally Inserted Central Catheter (PICC) in Neonatal Patients Caring for Temporary and Permanent Hemodialysis Catheters, e.g. Quinton or Perm Cath Declotting Central Venous Catheters with Alteplase, Partial or Total Occlusion References http://www.cdc.gov/ncidod/dhqp/gl_intravascular.html http://www.ihi.org/ihi/search/searchresults.aspx?searchterm=clabsi&searchtype=basic http://www.jointcommission.org/NR/rdonlyres/868C9E07-037F-433D-88580D5FAA4322F2/0/RevisedChapter_HAP_NPSG_20090924.pdf Pronovost, MD PhD, Peter, Needham, MD, PhD., Dale….An Intervention to Decrease Catheter-Related Bloodstream Infections in the ICU (Michigan Keystone Project), New England Journal of Medicine December 28, 2006; Vol. 355, #26. Maki DG, Mermel L, Genthner D, Hua S, Chiacchierini RP. An evaluation of BIOPATCH Antimicrobial Dressing compared to routine standard of care in the prevention of catheter-related bloodstream infection. Johnson & Johnson Wound Management, a division of ETHICON, INC., 2000. Data on file. Menyhay SZ, Maki DG. Disinfection of needleless catheter connectors and access ports with alcohol may not prevent microbial entry: the promise of a novel antiseptic-barrier cap. Infect Control Hosp Epidemiol. 2006;27:23-27. Ngo A. A Theory-based Intervention to Improve Nurses’ Knowledge, Self-efficacy, and Skills to Reduce PICC Occlusion. Journal of Infusion Nursing; Vol. 28, No. 3: pp 173-181. Oncu S et al. Central Venous Catheter-Related Infections: An Overview with Special Emphasis on Diagnosis, Prevention, and Management: The Internet Journal of Anesthesiology. 2003;Vol. 7, No. 1. Pyrek K. Battling Biofilm: Surface Science, Antimicrobials Help Combat Medical Device-Related Infections. Infection Control Today; Sept. 2002. http://www.infectioncontroltoday.com Ryder M. Catheter-Related Infections: It’s All About Biofilm. Topics in Advanced Practice Nursing eJournal. August 2005. Ryder M. The Role of Biofilm in Vascular Catheter-Related Infections. New Developments in Vascular Diseases: pp15-25. Timsit, J. Central vein catheter-related thrombosis in intensive care patients: incidence, risk factors, and relationships with catheter-related sepsis. Chest; July 1998. “To Err is Human: Building a Safer Health System.” Institute of Medicine. Quality of Health Care in America Project. 1999.