Chem. 31 * 9/15 Lecture

Chem. 31 – 3/11 Lecture
Announcements I
• Exam 1
– Grading error on p. 3 (problem 4); was graded as though 10 pts for entire problem – not just part a)
– That was the reason for getting 96 pts total and giving everyone 4 additional points
– Average = 77
• Cl lab report
– Due Today
• Homework Set 2 – Turn in
Problem 2.1
12
10
8
16
14
6
4
2
0
100s 90s 80s 70s 60s 50s <50
Название оси
Announcements II
• Quiz 3 Today
• Today’s Lecture – Chapter 7 “Advanced Equilibrium
Theory”
– Why Equilibirum Theory Can Fail
– Ionic Strength: What is it and how do we calculate it
– Replacement Equations: Activity and Activity Coefficients
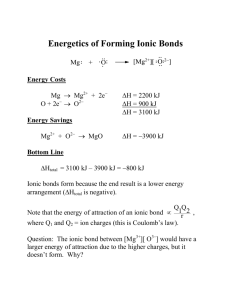
Chapter 7
“Adjustments” to Equilibrium Theory
• There are two areas where the general chemistry equilibrium theory can give wrong results:
– When the solution has high concentrations of ions
– When multiple, interacting equilibria occur
– I had planned a demonstration, but due to distance to class plus finding equipment, I’m skipping the demonstration this year
Demonstration – Slide 1
• Summary of Observation:
– Two saturated solutions of
MgCO
3 are prepared.
– One is prepared in water and the other is prepared in ~0.1 M NaCl.
– 5.0 mL of each solution was transferred (and filtered) into a beaker.
– 3.5 mL of 0.002 M HCl needed for saturated
MgCO
3 and 6.0 mL needed in 0.1 M NaCl
Saturated
MgCO
3
Saturated
MgCO
3 in
NaCl(aq)
Demonstration – Slide 2
• Did the moles of HCl used match expectations? and
Why did the solution containing NaCl need more HCl?
– First Question:
How many mL of HCl were expected?
MgCO
3
K sp
(s) Mg 2+ + CO since [Mg 2+ ] = [CO
10 -8 ) 0.5
3
2-
-4
3
2K
= 3.5 x 10 -8 = [Mg 2+ ][CO sp
3
2-
= 3.5 x 10 -8 T = 25°C
]
] (assuming no other reactions), [CO
M n(HCl) = (2 mol HCl/mol CO
0.001875 mmol HCl
Calculate V(HCl) = 0.001875 mmol HCl/[HCl]
= 0.001875 mmol HCl/0.002 mmol/mL = 0.935 mL
Actual V(HCl) > 1 mL
3
2] = (3.5 x
3
2)(1.87 x 10 -4 mmol/mL)(5.0 mL) =
• Conclusions
It takes more HCl than expected, so more CO
3
2-
Also, the NaCl increased the solubility of MgCO
3 dissolved than expected.
Demonstration – Slide 3
• What was the affect of the NaCl?
– More CO
3
2(and Mg 2+ ) was found to dissolve in the 0.10 M NaCl
• Why?
– The Na + and Cl ions stabilize CO
3 ions
2and Mg 2+
Ionic Strength Effects
Spheres Surrounding Ions
Low Ionic Strength High Ionic Strength
Ion
– dipole interaction
CO
3
2d+
H O
H d-
Mg 2+
Na +
Stronger ion
– ion interaction replaces ion - dipole
CO
3
2H
Mg 2+
O
H
Cl -
Ionic Strength
• Definition : m =
0.5*
S C i
Z i
2 where i is an ion of charge Z and molar concentration C.
• But What is Ionic Strength
– A measure which allows us to correct for ion – ion effects
• Examples:
– 0.10 M NaCl
– 0.010 M MgCl
2
– 0.010 M Ce(SO
4
)
2
Effects of Ionic Strength on Equilibria
• Equilibrium Equation Learned Previously:
– for reaction A ↔ B, K = [B]/[A]
• Replacement Equation:
– K =
– So what is
– A
X
– A
X
A / A
A
A
X
?
is the activity of X
= g
B
X
[X], where g strength
X
= activity coefficient
– The activity coefficient depends on the ionic
Determination of Activity
Coefficients
• Use of Debye-Hückel Equation: log g x
-
0 .
51
1
+
Z x
2
a x
305 m m
- where Z x
= ion charge, a x
= hydrated ion radius (pm)
- useful for 0.0001 M < m < 0.1 M
• Can also use Table 7-1 for specific m value
• Calculate g (Mg 2+ ) at m = 0.050 M a (Mg 2+ ) = 800 pm
Factors Influencing
g
• Ionic Strength: as m increase, g decreases
• Charge of Ion: a larger decrease in g occurs for more highly charged ions
• Size of Ion: Note: very small ions like Li + actually have large hydrated spheres ion
Gamma Plots
1.20
1.00
0.80
0.60
0.40
0.20
0.00
0.00
0.02
0.10
0.12
Li+
Ba2+
PO43-
Li + Rb +
0.04
0.06
Ionic Strength (M)
0.08
Hydrated sphere
Ionic Strength Effects on Equilibria
Qualitative Effects
• An increase in ionic strength shifts equilibria to the side with more ions or more highly charged ions
• Example Problems: (predict the shift as m increases)
– NH
3
(aq) + H
2
O(l) ↔ NH
– Cu 2+ + 4OH ↔ Cu(OH)
4
2-
– 2HSO
3
↔ S
2
O
3
2+ H
2
O(l)
– HSO
4
↔ SO
4
2+ H +
4
+ + OH -
Ionic Strength Effects
Effects on Equilibrium - Quantitative
• Calculate expected [Mg 2+ ] in equilibrium with solid MgCO
3 for cases both with and without NaCl.
– Go to Board