Right to Know - Stony Brook Medicine
advertisement

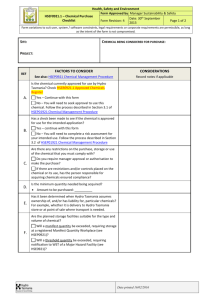
Hazard Communication/ Right-to-Know Environmental Health & Safety 4-6783 www.stonybrook.edu/ehs/healthcare 1 Training Outline 1. Hospital Safety Information Environment of Care Respiratory Protection Injury/Illness Reporting Reducing SB Medicine Staff Exposures Contaminated Sharps Injuries Formaldehyde (Formalin) Safety Glutaraldehyde/OPA Safety Hazardous Drugs (HD) Safety Ethylene Oxide (EtO) Safety Waste Anesthetic Gases (WAG) Safety Ergonomics Policy Environmental Awareness Transporting Infectious and Diagnostic Specimens 2. Hazard Communication/Right to Know 2 Environment of Care (EOC) Management Plans: • Each reference card has critical information • Posted in all patient care areas • Please review; you are responsible for knowing information on cards 3 Top 12 Ways to Insure EOC Compliance 1. Always wear your Stony Brook ID badge. 2. Know proper procedure for Major Chemical Spill response. – Call University Police at 911 or 631-632-3333 from cell phone. (Off sites call 911) 3. Know the location of your department’s Emergency Management Manual and Plan. 4. Know location of your unit’s disaster kit for power outages (flashlights, batteries, extension cord, duct tape, glow sticks). 5. Know how to shut off the oxygen supply valve. 4 Top 12 Ways to Insure EOC Compliance 6. Report building issues to Plant Operations at 4-2400. 7. Know proper fire response procedures (RACE). 8. Know locations of nearest fire alarm pull stations and how to use a fire extinguisher (PASS). 9. Secure your personal belongings. 10. Know how to access Safety Data Sheets, SDS, on-line (formerly referred to as MSDS). 11. Adhere to no smoking policy. 12. Report building related issues. (Tech Park: Contact Facilities Manager, 4-4380) 5 Respiratory Protection • SBUH requires all hospital employees, who come in contact with patients with known or suspect TB, other airborne pathogens or hazards, be annually fitted with a hospital approved N95 respirator. • If you experience significant weight gain or loss, dental or facial surgery, or other condition that may affect respirator fit, you must be re-fitted, even if it is within a year of your last fit test. 6 N95 Respirator- User Instructions 1. 2. 3. 4. 5. 6. Prior to wearing a respirator you must be medically cleared by Employee Health, and trained/fit tested by EH&S. Only wear the Type, Make, Model and Size respirator you were fitted with (e.g., N95, 3M 1860S). Keep fit test card with respirator information in your ID badge. Read user instructions. Inspect your respirator and conduct the User Seal Check prior to each use. Before you enter an isolation room that requires a respirator, put on your respirator in the corridor (not in the ante-room or patient room). For TB exposure, discard the N95 respirator when soiled or damaged. For SARS or smallpox exposure, discard after each use, unless directed otherwise. 7 N95 - No Interfering Facial Hair • Anyone with interfering facial hair cannot be fit tested or wear a N95 respirator because it prevents a good seal around the face. • A small goatee or mustache that fits inside the respirator may be OK, but a full beard or substantial “stubble” is not. 8 N95 Respirators Do I need to Wear a Surgical Mask over the N95 Respirator? Typically no. The hospital approved N95 respirators (3M 1860 and the Moldex/Inovel 1500 series) are both rated as surgical masks. 9 N95 Annual Respirator Fit Testing For Residents: Fit testing will take place the day of Orientation. (If you are not able to get fit tested at Orientation, you can be fit tested at one of the open monthly sessions). ================================== Open monthly fit testing schedule is provided in the Hospital weekly announcements and on the Hospital’s intranet under “Hot Topics”. 10 Injury/Illness Reporting Report any work related injury/illness to your supervisor. If necessary, seek medical attention at Employee Health and Wellness. If life threatening or off hours, go to the ED. Complete an Employee Injury/Illness Report and fax to “9”-706-4230. Contact the Accident Reporting System (ARS) at 888-800-0029. 11 Contaminated Sharps Injuries How can we prevent future injuries? 12 Reducing Contaminated Sharps Injuries Use a device with a safety, if available. If you are unsure how to use a device, seek guidance. Actively participate in trials of new safety devices. Limit distractions and conversations -- Don't disturb a colleague while they are using a sharp. Avoid multiple attempts during phlebotomy, ABGs, IV catheter insertions, CV catheter placement, and lumbar punctures. Use blunt tip needles in OR. Use extreme care when suturing. 13 Reducing Contaminated Sharps Injuries Take your time, don't rush. "Cool off" after interpersonal conflict. Avoid passing. Immediately discard used sharp in sharps container. When a sharps containers is ¾ full, have someone contact Hospital Custodial Services at 4-1455. (Off-sites: call Bob Weniger at 4-4066). Report all injuries and complete an Employee Injury/Illness Report and Sharps Injury Log. 14 Formaldehyde/Formalin Safety -human carcinogen- When using specimen containers with formalin: • • • • Minimize length of time containers are open Avoid spillage Clean up any spillage immediately Wear PPE – nitrile gloves 15 Glutaraldehyde and Cidex OPA Safety Glutaraldehyde and Cidex OPA – eye, skin, respiratory tract irritant How to minimize exposure: • • • • • Use appropriate ventilation: GUS Wear PPE (gloves, gown and eye protection). Pour carefully. Keep containers closed when not in use. Use Glute-out neutralizer prior to drain disposal and for spills. 16 Hazardous Drugs (HD) Safety Glutaraldehyde and Cidex OPA – • Look for “HD Precautions” sign on in-patient eye, skin, respiratory tract irritant room doors • Wear appropriate PPE Anticipation of hand exposure – wear double nitrile gloves, change out after 30 minutes For chemo and for anticipation of body splash – wear chemo gown • Avoid crushing or cutting HD tablets • Review HD Management policy, EC:0048 Hazardous Drug - as defined by NIOSH (National Institute of Occupational Health & Safety), any drug identified by at least 1 of the following 6 criteria: carcinogenicity, teratogenicity or other developmental toxicity, reproductive toxicity in humans, organ toxicity at low doses in humans or animals, genotoxicity, or new drugs that mimic existing HDs in structure or toxicity. Include drugs for cancer chemotherapy, antiviral drugs, hormones, some bioengineered drugs and other miscellaneous drugs and are identified on NIOSH’s List of Antineoplastic and Other Hazardous Drugs in Healthcare Settings. 17 Ethylene Oxide (EtO) Safety • EtO is a flammable, colorless gas used to sterilize surgical equipment • Central Sterile Supply (CSS) uses EtO in closed system sterilizers/ aerators • 2 EtO abators works automatically and interface with sterilizer/aerators • CSS has gas alarm system with master alarm panel outside EtO room with visual and audible alarms – 3 separate EtO monitors, in EtO Room and by each abator 18 Waste Anesthetic Gases (WAG) Safety • WAG = nitrous oxide and halogenated anesthetics (e.g., halothane, enflurane, isoflurane, desflurane). – exposure from leakage of patient's anesthetic breathing circuit during delivery of anesthetic and exhalations of patients recovering from anesthesia • Use anesthesia delivery units with gas scavenging as per manufacturer’s instructions. • Face masks used for administrating inhaled anesthetics should be available in variety of sizes, pliable, provide effective seal to prevent leakage, and positioned on the patient’s face properly. • Wear PPE during spills of liquid anesthetic agents (gloves, goggles, face shields) 19 Ergonomics Policy Ergonomics: EH&S is responsible for managing the Ergonomics Program, in consultation with Employee Health & Wellness science of human work focusing on designing work stations, tools & tasks for safety, efficiency & comfort. Musculoskeletal Disorders: (SDS) result from bodily reactions due to bending, climbing, crawling, reaching, twisting, overexertion, or repetitive motion. Injuries can occur to muscles, nerves, tendons, ligaments, joints, cartilage, and spinal discs in back, neck shoulder, elbow, wrist or hand. 20 Ergonomics Policy Reporting: Employees need to report work related injuries to their supervisor. Incident Trending: when a trend of MSD injuries is identified in an area, a hazard assessment will be conducted. Response: Controls may be used to reduce hazards Training: General ergonomics awareness will be provided by EH&S during Orientation, recertification classes and online. 21 Environmental Awareness What happens to waste that is poured down the drain? It goes to the Sewage Treatment Plant at the University and then into the Long Island Sound. Sewage Treatment Plant at the University Remember: Do not put any hazardous waste down the drain. Long Island Sound Port Jefferson, NY 22 What is Chemical Hazardous Waste? • • • • Chemical hazardous waste can include medications, cleaning products, paints, solvents, acids/bases. Most hazardous wastes have been identified throughout the Hospital and are being collected. If you are unsure whether you are generating a hazardous waste or are disposing of a new chemical product, complete a Waste Determination (Admin P&P EC:0045). If you have any questions, contact EH&S at 4-6783. 23 Common Chemical Waste Containers You might see the following 4 waste disposal containers on the units: • Pharmacy Return Box – all unused or expired drugs • Black 2-gallon container – Partially Used Drugs with a HW label. • Locked Box (Critical Care areas) – Epinephrine, Propofol (Diprivan), Eptifibatide (Integrilin) and Nitroglycerin • Chemo container (Oncology areas) – Chemotherapy waste. 24 HW Regulated Medical Waste (Red Bag Waste) • Place the following in Red Bags: • All waste generated in diagnosis, treatment or immunization of humans: • Cultures and stocks • Human pathological waste excluding teeth • Human blood and blood products • Waste products must be “saturated” with blood or bodily fluids so that when squeezed produces free flowing fluid or flecks 25 Sharps Waste Place in Sharps Containers: • Needles • All Syringes with needles • Syringes without needles that have come in contact with blood or body fluids • Pipettes • Slides and cover slips • Scalpel blades • Glass test tubes • Disposable staples • Explanted orthopedic hardware 26 Transporting Infectious Substances • All Infectious Substances and Biological Specimens must be packed and shipped by specially trained employees. • Hazardous materials MUST be properly labeled and packed for shipment. • Training is required and available from EH&S (4-6783). 27 Hazard Communication/RTK OSHA & PESH OSHA Occupational Safety & Health Administration Oversees Hazard Communication Standard in the private sector PESH Public Employees Safety & Health Bureau Oversees Hazard Communication (RTK) Standard for public employees at the state and local level 28 Required Departmental Postings Employers must post a sign in every workplace to inform employees that they have a right to hazard information. 29 Employer Responsibilities • Notify you about your right to request information • Provide information within 72 hours • Maintain information • Provide education and training • Maintain records on exposures • Maintain labeling system • Provide hazard information to nonemployees 30 Employee Rights • Submit written requests for information • Refuse to work with a toxic substance if no reply received within 72 hours • Obtain access to University’s written Hazard Communication, Right to Know program • Can not be forced to waive any rights under the Law as a condition of employment • Can file complaints with Department of Labor or NYS Attorney General 31 Revised Haz Comm Standard Hazard Communication Standard has aligned with the UN Globally Harmonized System of Classification and Labeling of Chemicals (GHS): • Provides common approach to classifying chemicals and communicating hazard info on labels and safety data sheets (SDS) • Improves quality and consistency of hazard information, making it safer for workers by providing understandable information on handling and safe use of hazardous chemicals Major changes to Haz Comm Standard • New Hazard classifications • New Labeling requirements Changes – Signal words (Danger or Caution), pictograms, hazard statements, precautionary statements • Standardized Safety Data Sheets (SDS) 33 Previous Labeling - NFPA Safety Diamond Flammability Assign a number to reflect the degree of hazard to the worker Health Reactivity RATING SYSTEM: 0-4 The higher the number, the higher the hazard! Special Hazard Label all secondary containers with product and hazard information! 34 New GHS Labeling 35 New Pictograms and Hazard Classes on Labels and Safety Data Sheets 36 Safety Data Sheets (SDS) – Your Guide to Workplace Safety • Material Safety Data Sheets are now called Safety Data Sheets (SDS) • SDS will be uniform, with the same 16 sections in the same order • SDS will have: – Signal word (Danger or Warning) – Hazard Pictograms – Hazard Statements – Precautionary Statements 37 Accessing SDS & Chemical Inventories • SDS and departmental inventories are available on-line at Hospital intranet, “Inside SBUMC”, under “RESOURCES” or go to: http://asamsds.campus.stonybro ok.edu/ 38 Accessing Pharmaceutical SDS • On the Hospital Intranet page, scroll down to Resources. • Select drop down for Drug References and Select MicroMedex. • Type in the name of the drug in the search field and click “Search”. • Scroll down and click on the "Toxicology and Exposure Information" section. • Click on MSDS. 39 Safety Data Sheets (SDS) Look for the red arrows throughout the MSDS sections. Why are SDS important to me? Provides details on: 1. Hazards 2. How to be protected from injury or illness – What PPE to wear – Safe work practices – Exposure controls 3. What to do if a spill occurs 4. What to do if a coworker or I get exposed – First aid 40 SDS Review Review of buffered, a Sample SDS • Formalin Solution, neutral 10% – Sigma Aldrich 41 SDS Review 16 sections: 1. 2. 3. 4. 5. 6. Identification Hazards Identification Composition/Ingredients First Aid Measures Fire Fighting Measures Accidental Release Measures 7. Handling & Storage 8. Exposure Controls & Personal Protection 9. Physical & Chemical Properties 10. Stability & Reactivity 11. Toxicological Info. 12. Ecological Info. 13. Disposal Info. 14. Transport Info. 15. Regulatory Info. 16. Other Info. 42 SDS – Section 1 Section 1 - Identification In case of an emergency. 43 SDS – Section 2 Section 2 - Hazards Identification How can this product harm me if improperly handled? 44 2. Hazards ID – Pictograms, Hazard You need to know how Statement, Precautionary statement this chemical could harm you, if not handled properly 45 SDS – Section 3 46 SDS – Section 4 Section 4 – First Aid Measures What do I do if this chemical get on my skin, in my eyes, or if I breathe it in? 47 SDS – Section 5 Section 5 – Firefighting Measures 48 SDS – Section 6 What do I do for a release or spill ? 49 Spill Kits for Minor Chemical Spills Glutaraldehyde Spill Kits Located near GUS stations and areas where glutaraldehyde/Cidex OPA used Chemotherapy Spill Kits Located in areas where chemotherapy are prepared and administered Formalin Spill Kits Located in labs and areas using formalin Battery Acid Spill Kits Located in powered industrial truck charging areas and other non-alkaline battery areas. 50 Chemical Spill Response • Minor spills of less than a gallon can be cleaned up by trained staff using a spill kit (chemo spills: < 50 ml). • Major spills over 1 gallon, call University Police at 911 (cell: 631632-3333). – Offsites: call 911 If at any time during clean up of a minor spill, you need assistance, call 911 (631-632-3333). 51 SDS – Section 7 Section 7 – Handling & Storage How should I handle and store this product safely? 52 SDS – Section 8 Section 8-Exposure Controls & Personal Protection How much can I be exposed to without experiencing problems? 53 Section 8 – Personal Protection What must I wear to protect myself… gloves, goggles, respirator? 54 SDS – Section 9 Section 9 – Physical & Chemical Properties 55 SDS – Section 10 Section 10 – Stability & Reactivity 56 SDS – Section 11 Section 11 – Toxicological Information How can this product harm me? 57 SDS – Section 12 Section 12 – Ecological Information 58 SDS – Section 13 Section 13 – Disposal Considerations How should I dispose of this product safely? 59 SDS – Section 14 Section 14 – Transport Information What must I do if I need to ship this product? 60 SDS – Section 15 Section 15 – Regulatory Information 61 SDS – Section 16 Section 16 – Other Information Codes used in Section 3 62