Periodic Trends Worksheet: Chemistry Practice
advertisement
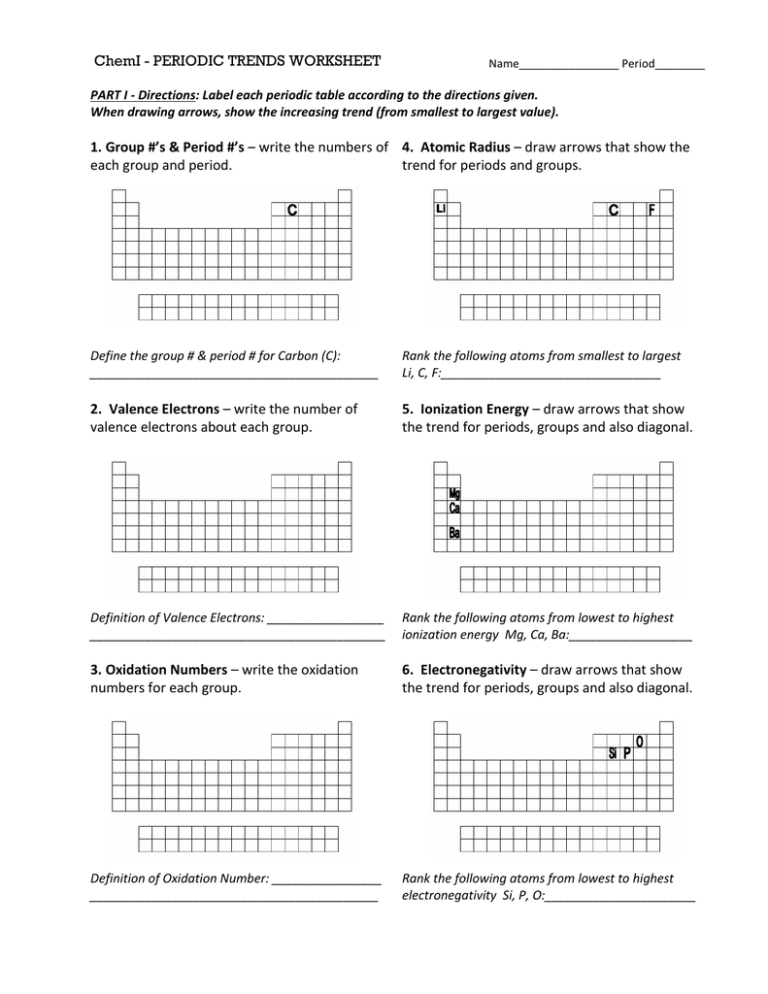
ChemI - PERIODIC TRENDS WORKSHEET Name________________ Period________ PART I - Directions: Label each periodic table according to the directions given. When drawing arrows, show the increasing trend (from smallest to largest value). 1. Group #’s & Period #’s – write the numbers of 4. Atomic Radius – draw arrows that show the each group and period. trend for periods and groups. Define the group # & period # for Carbon (C): __________________________________________ Rank the following atoms from smallest to largest Li, C, F:________________________________ 2. Valence Electrons – write the number of valence electrons about each group. 5. Ionization Energy – draw arrows that show the trend for periods, groups and also diagonal. Definition of Valence Electrons: _________________ ___________________________________________ Rank the following atoms from lowest to highest ionization energy Mg, Ca, Ba:__________________ 3. Oxidation Numbers – write the oxidation numbers for each group. 6. Electronegativity – draw arrows that show the trend for periods, groups and also diagonal. Definition of Oxidation Number: ________________ __________________________________________ Rank the following atoms from lowest to highest electronegativity Si, P, O:______________________ PART II - Directions: Using the information from your notes, answer the following questions. 1. What group are the most reactive metals located? 2. What group are the most reactive nonmetals located? 3. As you go from left to right across a period, the atomic size (decreases / increases). Why? 4. As you travel down a group, the atomic size (decreases / increases). Why? 5. As you go from left to right across a period, the first ionization energy generally (decreases / increases). Why? 6. As you travel down a group, the first ionization energy generally (decreases / increases). Why? 7. What element has the highest electronegativity? 8. What element has the lowest electronegativity? 9. Elements of Group 1 are called 10. Elements of Group 2 are called 11. Elements of Group 3-12 are called 12. As you go from left to right across the periodic table, the elements go from (metals / non-metals) to (metals / non-metals). 13. Group 17 elements are called 14. The most reactive element in Group 17 is 15. Group 18 elements are called 16. What sublevels are filling across the Transition Elements? 17. Elements within a group have a similar number of 18. Elements across a period have the same number of 19. An element that has lost or gained an electron is called 20. As you go down a group, the elements generally become (more / less) metallic. 21. The majority of elements in the periodic table are (metals / nonmetals). 22. Elements in the periodic table are arranged according to their 23. An element with both metallic and nonmetallic properties is called a



