File - JHS Chemistry and Physics
advertisement

Chapter 1 Objectives:
Explain why physics is the basic science.
Outline scientific methods.
Distinguish among observations, facts, hypotheses, laws, and principles.
Describe circumstances under which a hypothesis or law must be changed or abandoned.
Distinguish between the everyday meaning of theory and explain why the refinement of
theories is a strength in science.
Distinguish between a hypothesis that is scientific and one that is not.
Distinguish between science and technology.
Distinguish among science, art, and religion.
Chapter Terms:
fact
hypothesis
law
scientific method
theory
Chapter Outline Framework:
Scientific Measurements
o Size of the Earth
o Size of the Moon
o Distance to the Moon
o Distance to the Sun
o Size of the Sun
Mathematics: The Language of Science
The Scientific Method
The Scientific Attitude
Science, Art, and Religion
Physics: The Basic Science
In Perspective
Chapter 2 Objectives:
Describe Aristotle's concepts of natural and violent motion.
Describe Copernicus' Idea about Earth's motion.
Describe Galileo's contribution to the science of motion.
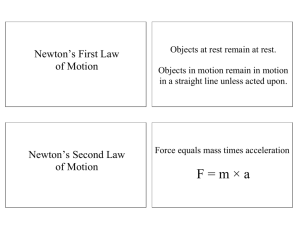
State Newton's first law of motion.
Distinguish among mass, volume, and weight, and their units of measurements.
Explain how something that is not connected to the ground is able to keep up with the moving
Earth.
Explain why a clothesline or wire that can easily support an object when strung vertically may
break when strung horizontally and supporting the same object.
Describe how the angle between vectors affects their resultant vector.
p
Chapter Terms:
inertia
force
mechanical equilibrium
equilibrium rule
Chapter Outline Framework:
Aristotle on Motion
Copernicus and the Moving Earth
Galileo and the Leaning Tower
Galileo's Inclined Planes
Newton's First Law of Motion
Net Force
The Equilibrium Rule
Support Force
Equilibrium of Moving Things
The Moving Earth
Chapter 3 Objectives:
Explain the idea that motion is relative.
Define speed and distinguish between instantaneous speed and average speed.
Distinguish between speed and velocity, and describe how to tell whether a velocity is
changing.
Define acceleration and give examples of its units.
Describe the motion of an object in free fall.
Describe the motion of an object thrown straight up and allowed to fall until it hits the
ground.
Determine the speed and the distance fallen at any time after an object is dropped from
rest, when air resistance is negligible.
Explain how graphs can be used to describe relationships among time, distance, and
speed.
Describe how air resistance affects the motion of falling objects.
Explain why acceleration is a rate of a rate.
Chapter Terms:
speed
velocity
acceleration
free fall
Chapter Formulas:
Speed = distance/time
Average Speed = total distance covered/time interval
Acceleration = change of velocity/time interval
Acceleration (along a straight line) = change in speed/time interval
Velocity acquired in free fall from rest: v = gt
Distance fallen in free fall from rest: v = ½ gt2
Chapter Outline Framework:
Motion is Relative
o Instantaneous Speed
o Average Speed
Velocity
Acceleration
o Accelerationon Galileo's Inclined Planes
Free Fall
o How Fast
o How Far
o How Quickly How Fast Changes
Hang Time
Chapter 4 Objectives:
State the relationship between acceleration and net force.
State the relationship between acceleration and mass.
State and explain Newton's second law of motion.
Describe the effect of friction on stationary and on moving objects.
Distinguish between force and pressure.
Explain why the acceleration of an object in free fall does not depend upon the mass of the
object.
Describe the effect of air resistance on a falling object.
Chapter Terms:
inertia
mass
weight
kilogram
newton
volume
force
friction
free fall
terminal speed
Chapter Formulas:
Acceleration ~ net force/mass
a ~ Fnet /m
F = mg
Chapter Outline Framework:
Force Causes Acceleration
Friction
Mass and Weight
Mass Resists Acceleration
Newton's Second Law of Motion
When Acceleration is g---Free Fall
When Acceleration is less than g---Non-Free Fall
Chapter Objectives:
Define force as part of an interaction.
State Newton's third law of motion.
Given an action force, identify the reaction force.
Explain why the accelerations caused by an action force and by a reaction force do not
have to be equal.
Explain why an action force is not canceled by the reaction force.
Describe the horse-cart problem.
Explain why you cannot touch without being touched.
Chapter Terms:
vector
vector quantity
scalar quantity
resultant
Chapter Outline Framework:
Forces and Interactions
Newton's Third Law of Motion
o Defining Your System
o Action and Reaction on Different Masses
Vectors
o Force Vectors
o Velocity Vectors
o Components of Vectors
Chapter Objectives:
Define momentum.
Define impulse and describe how it affects changes in momentum.
Explain why an impulse is greater when an object bounces than when the same object
comes to a sudden stop.
•Give an example of how the vector nature of momentum affects the law of conservation of
momentum.
Chapter Terms:
momentum
impulse
relationship of impulse and momentum
conservation of momentum
elastic collision
inelastic collision
Chapter Formulas:
mv(before event)= mv(after event)
Chapter Outline Framework:
Momentum
Impulse
Impulse Changes Momentum
o Case 1: Increasing Momentum
o Case 2: Decreasing Momentum Over a Long Time
o Case 3: Decreasing Momentum Over a Short Time
o
o
o
o
Bouncing
Conservation of Momentum
Collisions
More Complicated Collisions
Chapter Objectives:
Define and describe work.
Define and describe power.
Define mechanical energy.
Define potential energy.
Define kinetic energy and describe the work-energy theorem.
State the law of conservation of energy.
Describe simple machines and mechanical advantage.
Explain why no machine can have an efficiency of 100%.
Describe the role of energy in living organisms.
Chapter Terms:
work
energy
kinetic energy
potential energy
work-energy theorem
conservation of energy
machine
conservation of energy for machines
efficiency
Chapter Formulas:
W = Fd
Power = work/time
Kinetic Energy = Ke = ½ mv2
Work = ΔKe
Chapter Outline Framework:
Work
Power
Mechanical Energy
o Potential Energy
o Kinetic Energy
o Work-Energy Theorem
Conservation of Energy
Machines
Efficiency
Comparison of Kinetic Energy and Momentum
Sources of Energy
Energy for Life
Chapter Objectives:
Distinguish between rotate and revolve.
Describe rotational speed.
Give examples of centripetal force.
Describe the motion of an object if the centripetal force acting on it ceases.
Explain why centrifugal force is "fictitious."
Describe how a simulated gravitational acceleration can be produced.
•Define and describe torque.
Describe the condition for one torque to balance another.
Describe center of gravity.
Describe center of mass
Describe how to find the center of gravity of an irregularly shaped object.
Given the location of the center of gravity of an object and the position and direction of the
forces on it, tell whether the forces will produce rotation.
Describe on what the rotational inertia of an object depends.
Define angular momentum and describe the conditions under which it (a) remains the
same and (b) changes.
Give an example in which rotational speed changes but angular momentum does not.
Chapter Terms:
tangential speed
rotational speed
rotational inertia
torque
center of mass (CM)
center of gravity (CG)
equilibrium
centripetal force
dentrifugal force
angular momentum
conservation of angular momentum
Chapter Formulas:
Torque = lever arm x force
Chapter Outline Framework:
Circular Motion
Rotational Inertia
Torque
Center of Mas and Center of Gravity
o Locating the center of Gravity
o Stability
Centripetal Force
Centrifugal Force
Centrifugal Force in a Rotating Reference Frame
Simulated Gravity
Angular Momentum
Conservation of Angular Momentum
Chapter Objectives
Explain Newton's idea of why the apple falls to Earth.
Explain why the moon does not fall to Earth.
Explain how Earth is falling.
State Newton's law of universal gravitation.
Explain the significance of an inverse-square law.
Explain the connection between gravitation and the idea that the universe may stop
expanding and begin to contract.
Describe the gravitational field outside Earth.
Describe the gravitational field inside Earth.
Explain why an astronaut in Earth orbit seems weightless even though there is a
gravitational force.
Explain ocean tides.
Give examples of tides other than those in water.
Describe black holes.
Chapter Terms:
law of universal gravitation
inverse-square law
weightlessness
spring tide
neap tide
gravitational field
black hole
Big Bang
Chapter Formulas:
F= G
m1m2
d2
Chapter Outline Framework:
The Universal Law of Gravity
The Universal Gravitational Constant, G
Gravity and Distance; The Inverse Square Law
Weight and Weightlessness
Ocean Tides
o Tides in the Earth and Atmosphere
o Tides on the Moon
Gravitational Fields
Gravitational Field Inside a Planet
Einstein's Theory of Gravitation
Black Holes
Universal Gravitation
Chapter Objectives:
Explain how the speed of a satellite in circular orbit around Earth is related to the distance
an object falls in the fist second due to gravity.
Explain why the force of gravity does not cause a change in the speed of a satellite in
circular orbit.
Describe how the speed of a satellite changes in different portions of an elliptical orbit.
Apply the energy conservation law to describe changes in the PE and KE of a satellite in
different portions of an elliptical orbit.
Determine the vertical speed required to ensure a projectile can "escape" Earth.
Chapter Terms:
projectile
parabola
satellite
ellipse
escape speed
Chapter Outline Framework:
Projectile Motion
o Projectiles Lanched Horizontally
o Upwardly Launched Projectiles
Fast-Moving Projectiles -- Satellites
Circular Satellite Orbits
Elliptical Orbits
World Monitoring by Satellite
Kepler's Laws of PlanetaryMotion
Energy Conservation and Satellite Motion
Escape Speed
Chapter Objectives:
Describe atoms and elements.
Compare the ages of atoms to the ages of the materials they compose.
Give examples that illustrate the small size of atoms.
State evidence for the existence of atoms.
Describe molecules.
Describe compounds.
Identify and describe the building blocks that make up the atom.
Explain the organization of the periodic table.
Describe the solid, liquid, gaseous, and plasma states of matter.
Chapter Terms:
atom
Brownian motion
atomic nucleus
electron
proton
neutron
atomic number
atomic mass unit (amu)
Chapter Outline Framework:
The Atomic Hypothesis
The Elements
Atomic Imagery
The Electron
The Atomic Nucleus
The Proton
The Neutron
Quarks
Elements, Compounds, and Mixtures
Molecules
Antimatter
Dark Matter
isotpes
periodic table
comound
molecule
chemical reaction
mixture
quantum mechanics
antimatter
dark matter
Chapter Objectives:
Describe the structure of crystals.
Define density and explain why it is the same for different amounts of the same material.
Distinguish between an elastic material and an inelastic material, and describe Hooke's
law.
Explain why the center of a horizontal steel girder need not be as wide as the top and
bottom.
Describe the relationship among linear growth, surface area growth, and volumetric
growth.
Chapter Terms:
atomic bonding
density
elasticity
Hooke's Law
scaling
Chapter Formulas:
Density = mass/volume
weight density = weight/volume
Hook's Law F = kΔx (where k is the spring constant)
Chapter Outline Framework:
Müller's Micrograph
Crystal Structure
Density
Elasticity
Tension and Compression
Arches
Scaling
Chapter Objectives:
Describe what determines the pressure of a liquid at any point.
Explain what causes a buoyant force on an immersed or submerged object.
Relate the buoyant force on an immersed or submerged object to the weight of the fluid it
displaces.
Describe what determines whether an object will sink or float in a fluid.
Given the weight of a floating object, determine the weight of fluid it displaces.
Describe how Pascal's principle can be applied to increase the force of a fluid on a
surface.
Chapter Terms:
pressure
buoyant force
Archimedes' principle
principle of flotation
Pascal's principle
surface tension
capillarity
Chapter Formulas:
pressure = force/area
liquid pressure = weight density x depth
Chapter Outline Framework:
Pressure
Pressure in a Liquid
Buoyancy
Archimedes' Principle
What Makes an Object Sink or Float?
Flotation
Pascal's Principle
Surface Tension
Capillarity
Chapter Objectives:
Explain why the molecules in Earth's atmosphere neither escape nor settle to the ground.
Describe the source of atmospheric pressure.
Explain why water cannot be raised higher than 10.3m with a vacuum pump.
Describe the aneroid barometer.
Describe the relationship between pressure and density for a given amount of a gas at a
constant temperature.
Explain what determines whether an object will float in air.
Describe the relationship between the speed of a fluid at any point and the pressure at that
point, for steady flow.
Describe some applications of Bernoulli's principle.
Chapter Terms:
atmospheric pressure
barometer
Boyle's law
Archimedes' principle for air
Bernoulli's principle
plasma
Chapter Formulas:
P1V1 = P2V2
Chapter Outline Framework:
The Atmosphere
Atmospheric Pressure
o Barometers
Boyle's Law
Buoyancy of Air
Bernoulli's Principle
o applications of Bernoulli's Principle
Plasma
o Plasma in the Everyday World
o Plasma Power
Chapter Objectives:
Define temperature in terms of KE and describe the common temperature scales.
Define heat.
Define thermal equilibrium.
Distinguish between internal energy and heat.
Describe how the quantity of heat that enters or leaves a substance is measured.
Compare the specific heat capacities of different substances.
Describe how water's high specific heat capacity affects climate.
Give examples and applications of thermal expansion of solids.
Describe the behavior of water as it is heated from 0oC to 15oC.
Chapter Terms:
temperature
absolute zero
heat
internal energy
specific heat capacity
Chapter Outline Framework:
Temperature
Heat
Specific Heat Capacity
Thermal Expansion
o Expansion of Water
Chapter Objectives:
Explain conduction and its effects.
Distinguish between conduction and convection.
Explain how heat can be transmitted through empty space.
Given the color and shininess of two objects, predict which is likely to absorb radiant
energy more easily.
Compare the ability of an object to emit radiant energy with its ability to absorb radiant
energy.
Relate the temperature difference between an object and its surroundings to the rate at
which it cools.
Describe global warming and Earth's greenhouse effect.
Chapter Terms:
conduction
convection
radiation
Newton's Law of Cooling
greenhouse effect
solar constant
solar power
Chapter Outline Framework:
Conduction
Convection
Radiation
o Emission of Radiant Energy
o Absorption of Radiant Energy
o Reflection of Radiant Energy
o Cooling at Night by Radiation
The Greenhouse Effect
Solar Power
Controlling Heat Transfer
Chapter Objectives:
Describe the concept of absolute zero.
State the fist law of thermodynamics and relate it to energy conservation.
Describe adiabatic processes and cite examples.
State the second law of thermodynamics.
Define the ideal efficiency of a heat engine in terms of input and output temperatures.
Explain how order tends to disorder.
Define entropy and give examples.
Chapter Terms:
thermodynamics
absolute zero
internal energy
first law of thermodynamics
adiabatic process
temperature inversion
second law of thermodynamics
heat engine
entropy
Chapter Outline Framework:
Absolute Zero
Internal Energy
First Law of Thermodynamics
Adiabatic Processes
Meterology and the First Law
Second Law of Thermodynamics
o heat engines
Order Tends to Disorder
Entropy
Chapter Objectives:
Describe the period of a pendulum.
Describe the characteristics and properties of waves.
Describe wave motion.
Describe factors that affect the speed of a wave.
Distinguish between transverse waves and longitudinal waves.
Distinguish between constructive and destructive interference.
Describe how a standing wave occurs.
Describe the Doppler effect for sound and relate it to the blue and red shifts for light.
Describe bow waves.
Describe sonic booms.
Chapter Terms:
sine curve
amplitude
wavelength
frequency
hertz
period
wave speed
transferse wave
longitudinal wave
interference pattern
standing wave
Doppler effect
bow wave
sock wave
sonic boom
Chapter Formulas:
wave speed = wavelength x frequency
Chapter Outline Framework:
Vibration of a Pendulum
Wave Description
Wave Motion
Wave Speed
Transverse Waves
Longitudinal Waves
Interference
o Standing Waves
Doppler Effect
Bow Waves
Shock Waves
Chapter Objectives:
Relate the pitch of a sound to its frequency.
Describe the movement of sound through air.
Compare the transmission of sound through air with that through solids, liquids, and a
vacuum.
Describe factors that affect the speed of sound.
Describe loudness and sound intensity.
Give examples of forced vibration.
Describe natural frequency.
Describe resonance.
Describe how sound waves interfere with one another.
Describe beats.
Chapter Terms:
infrasonic
untrasonic
compression
rarefaction
forced vibration
natural frequency
resonance
beats
carrier wave
modulation
amplitude modulation (AM)
frequency modulation (FM)
Chapter Outline Framework:
Origin of Sound
Nature of Sound in Air
Media that Transmit Sound
Speed of Sound in Air
Reflection of Sound
Refraction of Sound
Energy in Sound Waves
Forced Vibrations
Natural Frequency
Resonance
Interference
Beats
Chapter Objectives:
Describe the characteristics of musical tones in terms of pitch. loudness, and quality.
Differentiate between graphical representations of music and noise
Differentiate between sound intensity and loudness.
Describe what determines the musical quality of a note.
Identify the three principle classes of musical instruments.
Discuss Fourier's discoveries abaout complex periodic wave patterns.
Chapter Terms:
pitch
intensity
loudness
quality
partial tone
fundamental frequency
harmonic
Fourier analysis
Chapter Outline Framework:
Pitch
Soiund Intensity and Loudness
Quality
Musical Instruments
Fourier Analysis
Compact Discs
Chapter Objectives:
Describe electrical forces between objects.
Explain how an object becomes (a) positively charged and (b) negatively charged.
Describe Coulomb's law.
Distinguish between a conductor and an insulator.
Describe how an insulator can be charged by friction and by contact.
Describe how a conductor can be charged without contact.
Describe how an insulator can be charged by charge polarization.
Chapter Terms:
electricity
electrostatics
conservation of charge
coulomb's law
couloumb
conductor
insulator
electrically polarized
electric field
electrid potential energy
electric potential
capacitor
Chapter Formulas:
Voltage = electric potential energy/amount of charge
Chapter Outline Framework:
Electrical Forces
Electric Charges
Conservation of charge
Coulomg's Law
Conductors and Insulators
o Semiconductors
o Superconductors
Charging
o Charging by Friction and Contact
o Charging by Induction
Charge Polarization
Electric Field
o Electric Shielding
Electric Potential
Electric Energy Source
Van de Graaff Generator
Chapter Objectives:
Describe the flow of electric charge.
Describe what is happening inside a current-carrying wire.
Give examples of voltage sources that can maintain a potential difference in a circuit.
Describe the factors that affect the resistance of a wire.
Describe Ohm's law.
Explain the causes of electric shock.
Distinguish between DC and AC and describe how AC is converted to DC.
Compare the drift speed of conducting electrons in a current-carrying wire to the signal
speed of changes in current.
Compare the motion of electrons in a wire carrying AC to the flow of energy through the
wire.
Relate the electric power used by a device to current and voltage.
Chapter Terms:
potential difference
electric current
electrical resistance
superconductor
Ohm's law
direct current (dc)
alternating current (ac)
electric power
series circuit
parallel circuit
Chapter Forumlas:
Chapter Outline Framework:
Flow of Charge
Electric current
Voltage Sources
Electrical Resistance
Ohm's Law
o Ohm's Law and Electric Shock
Direct Current and Alternating Current
o Converting ac to dc
Speed and Source of Electrons in a Circuit
Electric Power
Electric Ciruuits
o Series Circuits
o Parallel Circuits
o Parallel Circuits and Overloading
o Safety Fuses
Chapter Objectives:
Compare and contrast magnetic poles and electric charges.
Use iron filings to interpret the strength of a magnetic field at different points near a
magnet.
Relate the motion of electrons within a material to the ability of the material to become a
magnet.
Describe what happens to the magnetic domains of iron in the presence of a strong
magnet.
Describe the magnetic field produced by a current-carrying wire.
Describe how a magnetic field exerts a force on a charged particle in the field.
Describe some practical applications of a magnetic field exerting a force on a currentcarrying wire.
Describe how a galvanometer and a motor work.
Suggest possible causes for Earth's magnetic field.
Chapter Terms:
magnetic force
magnetic field
magnetic domains
electromagnet
Chapter Outline Framework:
Magnetic Forces
Magnetic Poles
Magnetic Fields
Magnetic Domains
Electric Currents and Magnetic Fields
Electtromagnets
Magnetic Force on Moving Charged Particles
Magnetic Force on Current-CarryingWires
o Electric Meters
o Electric Motors
o Earth's Magnetic Field
o Cosmic rays
o Biomagnetism
Chapter Objectives:
Describe how voltage is induced in a coil of wire.
State and explain Faraday's law.
Describe how a generator works.
Compare and contrast motors and generators.
Describe how a transformer works.
Explain why transformers are used for transmission of electric power.
Relate the magnitude and direction of an induced electric field to the inducing magnetic
field, and vice versa.
Describe electromagnetic waves.
Chapter Terms:
Electromagnetic induction
Faraday's law
generator
transformer
Maxwell's counterpart to Faraday's law
Chapter Outline Framework:
Electromagnetic Induction
Faraday's Law
Generators and Alternating Current
Power Production
o Turbogenerator Power
o Transformers
Self-induction
Power Transmission
o Field Induction
In Perspective
Chapter Objectives:
Describe the dual nature of light.
Explain why it is difficult to measure the speed of light.
Describe the relationship among light, radio waves, microwaves, and X-rays.
Explain how the frequency of light affects what happens when it enters a substance.
Describe opaque materials.
Describe solar and lunar eclipses.
Describe the evidence that suggests light waves are transverse.
Describe 3-D vision.
Chapter Terms:
electromagnetic wave
electromagnetic spectrum
transparent
opaque
shadow
umbra
penumbra
solar eclipse
lunar eclipse
Chapter Outline Framework:
Electromagnetic Waves
o Electromagnetic Wave Velocity
o The Electromagnetic Spectrum
Transparent Materials
Opaque Materials
o Shadows
Seeing Light: The Eye
Chapter Objectives:
Explain why white and black are not true colors.
Describe how the reflection of light affects an object's color.
Describe what determines whether a material reflects, transmits, or absorbs light of a
particular color.
Describe white light.
Explain that color television tubes produce only red, green and blue light.
Define complementary colors.
Describe color mixing by subtraction and by addition.
Explain why the sky is blue, why sunsets are red, and why water is greenish-blue.
Explain how a spectrum can be used to identify the presence of an element.
Chapter Terms:
additive primary colors
complementary colors
subtractive primary colors
Chapter Outline Framework:
Selective Reflection
Selective Transmission
Mixing colored Light
o Complementary Colors
Mixing colored Pigments
why the Sky is Blue
Why Sunsets are Red
Why Clouds are White
Why Water is Greenish-Blue
Chapter Objectives:
Describe what happens to light when it strikes different materials.
Describe the law of reflection.
Explain why a mirror forms a virtual image.
Describe diffuse reflection.
Give examples of ways to control reflected sound.
Explain the change in direction of a wave when it crosses a boundary between media.
Describe the effects of refraction of sound waves.
Describe the effects of refraction of light.
Explain how mirages are formed.
Explain how a prism separates white light into colors.
Describe how a rainbow is formed.
Describe total internal reflection, its effects, and its applications.
Chapter Terms:
reflection
refraction
Fermat's principle of least time
law of reflection
diffuse reflecton
critical angle
total internal reflection
converging lens
diverging lens
virtual image
real image
aberration
Chapter Outline Framework:
Reflection
{romco[;ef :Least Time
Law of Reflection
o Plane Mirrors
o Diffuse Reflection
Refraction
cause of Refraction
o Dispersion
o Rainbows
Total Internal Refraction
Lenses
o Image Formation by a Lens
Chapter Objectives:
Explain why, after passing through a narrow opening, water waves have curved wave
fronts.
Describe the causes of visible diffraction of waves.
Describe the causes of visible bright and dark interference fringes of light.
Describe Young's interference experiment.
Explain the causes of the bright and dark bands that appear when monochromatic light is
reflected from a thin material.
Explain why colors shine from soap bubbles and gasoline slicks on a wet surface.
Describe how laser light is different from light from an ordinary lamp.
Explain how holograms are formed.
Chapter Terms:
Huygen's principle
diffraction
interference
polarization
hologram
Chapter Outline Framework:
Huygen's Principle
Diffraction
Interference
o Single-color Thin Film Interference
o Interference Colors by Reflection from THin Films
Polarization
o Three-Dimensional Viewing
Holography
Chapter Objectives:
Compare and Contrast Emission and Absorption Spectra
Describe the process of incandescence
Describe the proccess of fluorescence
Describe the process of phosphorescence
Describe how laser light is different from light from an ordinary lamp.
Explain how holograms are formed.
Describe the process of excitation
Chapter Terms:
exicitation
emission spectrum
spectroscope
incandescence
absorption spectrum
fluorescence
phosphorescence
laser
Chapter Outline Framework:
Excitation
o Emission Spectra
Incandescence
o Absorption Spectra
Fluorescence
o Fluorescent Lamps
Phosphorescence
Lasers
Chapter Objectives:
Describe the historical development of the Quantum Theory.
Explain the relation of quantization and Plank's constant.
Describe the photoelectric effect.
Discuss light as waves and as particles.
Describe the double slit experiment and explain its significance.
Explain the uncertainty principle
Discuss the concept of complementarity.
Chapter Terms:
quantum theory
Planck's constant
o h = 6.6 x 10-34 joule-second
photoelectric effect
uncertainty principle
complementarity
Chapter Outline Framework:
Birth of the Quantum Theory
Quantization and Planck's Constant
Photoelectric Effect
Wave-Particle Duality
Double-Slit Experiment
Particles as Waves; Electron Diffraction
Uncertainty Principle
Complementarity
Chapter Objectives;
Describe the experiments that led to the discovery of the atomic nucleus
Discuss how atomic spectra provide clues to atomic structure
Describe the Bohr Model fo the Atom
Compare the relative sizes of atoms.
Explain quantized energy levels in atoms
Describe the main points of quantum mechanics.
Describe the correspondence principle.
Chapter Terms:
Ritz combination principle
quantum mechanics
Shrödinger's wave equation
correspondence principle
Chapter Outline Framework:
Discovery of the Atomic Nucleus
Atomic Spectra: Clues to Atomic Structure
Bohr Model fo the Atom
Relative Sizes of Atoms
Explanation of Quantized Energy Levels; Electron Waves
Quantum Mechanics
Correspondence Principle
Chapter Objectives:
Explain the Relation of x-rays and radioactivity
Describe the differences and similarities among alpha, beta, and gamma rays
Describe the forces that hold the nucleus together.
Describe the particles that form the nucleus
Describe how the nuclei of isotopes differ
Explain the difference between stable and radioactive isotopes.
Explain why atoms are radioactive.
Define half-life.
Describe natural and artificial transmutation of elements.
Explain several methods for dating objects.
Descxribe the effects of radiation on humans.
Chapter Terms:
x-ray
alpha particle
beta particle
gamma ray
nucleon
quarks
isotopes
atomic number
atomic mass number
half-life
transmutation
Chapter Outline Framework:
X-Rays and Radioactivity
Alpha, Beta, and Gamma Rays
The Nucleus
Isotopes
Why Atoms Are Radioactive
Half-Life
o Radiation Detectives
Natural Transmutation of Elements
Artificial Transmutation of Elements
Radioactive Isotopes
Carbon Dating
Uranium Dating
Effects of radiation on Humans
o Radiation Damage
Chapter Objectives:
Compare and contrast nuclear fission and nuclear fusion.
Describe nuclear fission reactions.
Explain the role of plutonium in nuclear fission.
Describe the Breeder reacton
Discuss the advantages and limitations of nuclear fission and nuclear fusion as energy
sources.
Chapter Terms:
nuclear fission
chain reaction
critical mass
Breeder reactor
nuclear fusion
thermonuclear fusion
Chapter Outline Framework:
Nuclear Fission
Nuclear Fission Reactions
Plutonium
The Breeder Reactor
Fission Power
Mass-Energy Equivalence
Nuclear Fusion
Controlling Fusion
Chapter Objectives:
Describe the concept of relative motion.
Describe the Michelson-Morley experiment.
List the postulates of the Special Theory of Relativity.
Discuss how the Special Theory of Relavitivity applies to time dilation, length contraction,
space time, space travel, and length contraction.
Define relativistic momentum.
Chapter Terms:
frame of reference
postulates of the special theory of relativity
simultaneity
spacetime
time dilation
length contraction
mass-energy equivalence
Chapter Outline Framework:
Motion is Relative
Michelson-Morley Experiment
Postulates of the Special Theory of Relativity
Simultaneity
Spacetime
Time Dilation
The Twin Trip
Addition of Velocities
Space Travel
Length Contraction
Relativistic Momentum
Chapter Objectives:
Describe the principle of equivalence.
Explain how gravity bends light
Discuss the relation of gravity and time.
Discuss the relation of gravity and space.
Discuss gravity as a dimension of space.
Explain gravitational waves.
Compare and contrast Newton's and Einstein's concepts of gravity.
Chapter Terms:
general theory of relativity
principle of equivalence
gravitational red shift
geodesic
gravitational wave
Chapter Outline Framework:
Principle of Equivalence
Bending of Light by Gravity
Gravity and TIme: Gravitational Red Shift
Gravity and Space: Motion of Mercury
Gravity, Space, and a New Geometry
Gravitational Waves
Newtonin and Einsteininian Gravitation
The Physics Classroom
Fear of Physics
Jefferson Lab
The Applet Collection
The Particle Adventure
Physics Central
Physics Coloring Book
Revolutions In Science
Physics Student Websites and Hotlists
Chemistry and Physics Animations
Online Science Labs
Mechanical Universe Videos