COI in Research IP - RA Training
advertisement

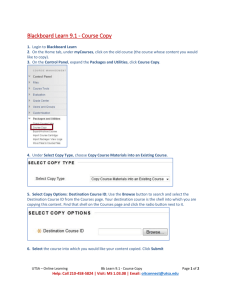
COI 101: What is Conflict of Interest and How Can it Affect You? Jennifer Morales Research Compliance Specialist Office of Research Integrity & Compliance What is Conflict of Interest? A situation in which financial or other personal considerations may compromise or have the appearance of compromising an employee's professional judgment in administration, management, teaching, research or any other professional activities. Examples of Possible COIs or those Requiring Management Plans (not inclusive) Failure of the faculty member/researcher to fulfill university responsibilities (e.g., holding classes, advising students, conducting research…), due to involvement in external activities. Sponsored projects or technology licensing arrangements in which any of the involved investigators (or members of their immediate families) have employment or consulting arrangements and significant financial interests in the sponsor, or with subcontractors, vendors, or collaborators. Using university resources to conduct research that is sponsored by an entity in which the faculty member/researcher or his/her family member has a significant financial interest. Serving as a consultant or on the board of directors or major advisory committee of an external for-profit entity which sponsors the faculty member's/researcher's research or provides gift funds for the researcher or his/her department. Hiring university students in consulting activities or a company in which the faculty member/researcher has financial interests. If the student's thesis/dissertation research is supervised by the faculty member, the conflict of interest situation may not be manageable. Examples of Possible COIs or those Requiring Management Plans (not inclusive) While acting in the context of his/her university duties, making professional referrals to or purchasing materials or services from a business in which an academic staff member or family member has a financial interest. Serving as president or CEO, or holding any other position that requires involvement in the day-to-day operations of a for-profit sponsor. Such a situation creates an unacceptable conflict of interest and/or commitment, which must be reduced in order to be manageable. Conducting clinical trials on a drug or device developed by the faculty member/researcher may be manageable if the research DOES NOT involve human research subjects. Equity (ownership) interest of the faculty member (or members of the immediate family) in a sponsor. Diverting research opportunities from the university to a consulting entity or business in which the faculty/researcher has a financial interest. Possible Conflict of Interest Situation – Funded Research ABC Company UTSA Undisclosed Consultant Agreement $75,000 fees & 25,000 shares Faculty/Researcher UTSA Resources/Lab Facilities Student Research Projects Research Data Publications & Possible Commercialization Grades/Graduation Possible Conflict of Interest Situation – Non Funded Research UTSA Undisclosed Consultant Agreement $75,000 fees & 25,000 shares Faculty/Researcher Student Research Projects UTSA Resources/Lab Facilities Research Data Publications & Possible Commercialization License Intellectual Property/ Provisional Patent Grades/Graduation ABC Company Guiding Principles The fact is that there will be conflicts. The term Conflict of Interest “implies” wrongdoing. Not all conflicts are necessarily impermissible and MOST are manageable. When human subjects are involved, there will be a higher level of scrutiny Complete and timely disclosure is essential Case by case analysis and management is crucial When conflicts of interest are handled appropriately Helps prevent loss of public trust Promotes objectivity in research Increases public funding of research Protects the reputation of the University as a credible research institution And…last but not least, It’s the LAW! COI Federal & State Regulations 42 CFR 50 Subpart F deals with grants and cooperative agreements 45 CFR Part 94 deals with contracts Texas Institutions should also see: Texas Government Code Chapter 572 Section 572.005 How does UTSA Deal With COI? First, a distinction was made between the following: 1. Institutional Conflict of Interest which is driven by Texas Statute (Chapter 572, Government Code) This Statute has different thresholds for reporting and does not define areas pertinent to research (i.e. human subjects, intellectual property, etc.) 2. Conflict of Interest in Research & Intellectual Property Follows federal regulations (CFR Title 42, Vol1 Part 50 Subpart F & CFR Title 45, Volume 1 Part 94 Section 94.4) Institutional Responsibilities as defined by Federal Regulations 1) 2) 3) 4) Maintain a written, enforced policy Inform Investigators of the policy, the regulations and their responsibilities Establish enforcement mechanisms and define sanctions for non-compliance Comply with the retention regulation – 3 years from date of submission of final expenditures report Institutional Responsibilities as defined by Federal Regulations Designate an Institutional Official to solicit and review disclosure statements provide guidelines to identify COI take action to ensure COI issues are managed, reduced or eliminated CCOI Structure Voting Members and one alternate for each as follows: 7 members, one from each College of the University One Community member not otherwise affiliated with the University Small Business Development Center representative Institutional Review Board representative Office of Research Integrity and Compliance Ex-officio Members, non-voting member and alternate from the following: Institutional Compliance and Risk Assessment Technology Transfer Office Office of Sponsored Programs Office of Vice President for Business Affairs Policy UTSA policy applies (and at times exceeds) the federal regulations and includes both funded and non-funded research. Components of Conflict of Interest Management Disclosure Review Management Monitoring Reporting Investigator Responsibilities Submit financial disclosure statements listing all Significant Financial Interests: That would reasonably appear to be affected by the research In entities whose financial interests would reasonably appear to be affected by the research Comply with all institutional requirements Who should disclose? The Principal Investigator AND any other person responsible for the design, conduct, or reporting of research or proposed research AND includes their Spouse/Domestic Partner, Dependent Children and Others in the Household Who does that include? RAs & Graduate RAs Sub-Contractors Sub-Recipients Lab Personnel Research Doctoral & Post Doctoral Students PIs Co-PIs Other Collaborators Includes their Spouse/Domestic Partner, Dependent Children and Others in the household Significant Financial Interest Anything of monetary value, including but not limited to, salary or other payments for services (e.g., consulting fees or honoraria); equity interests (e.g., stocks, stock options or other ownership interests); and intellectual property rights (e.g., patents, copyrights and royalties from such rights). The term does NOT include: Salary, royalties, or other remuneration from the applicant institution; Any ownership interests in the institution, if the institution is an applicant under the SBIR Program (including STTR); (UTSA Policy Differs) Income from seminars, lectures, or teaching engagements sponsored by public or nonprofit entities; (UTSA Policy Differs) Income from service on advisory committees or review panels for public or nonprofit entities; (UTSA Policy Differs) The term does NOT include: An equity interest that when aggregated for the Investigator and their spouse and dependent children, meets both of the following tests: 1)Does not exceed $10,000 in value as determined through reference to public prices or other reasonable measures of fair market value, and 2) does not represent more than a five % ownership interest in any single entity; or (UTSA Policy Differs) Salary, royalties or other payments that when aggregated for the Investigator and their spouse and dependent children over the next twelve months, are not expected to exceed $10,000. (UTSA Policy Differs) What is a Financial Conflict of Interest? UTSA adopted the NIH definition: A significant financial interest that could directly and significantly affect the design, conduct, or reporting of research. Significant Financial Interest vs. Financial Conflict Of Interest: A Significant Financial Interest (SFI) is not always a Financial Conflict of Interest (FCOI) An FCOI exists when a designated Institutional Official reasonably determines that an SFI could directly and significantly affect the design, conduct, or reporting of the research (usually with the recommendations of a COI Committee) Procedure Created and implemented a procedure for collecting Disclosure Forms st Annually on February 1 for all researchers, and others specified by Chair/Dean, whether research is funded or not funded Updated PRIOR to submitting a funding proposal or entering into a technology licensing arrangement, or any other contract that binds the University. Within 30 days of hire/appointment for all new faculty researchers or new staff on research projects. Electronic System An electronic system was purchased from ClickCommerce and was implemented on February 1, 2010 It is tied into the mandatory acknowledgements which ALL employees are currently required to complete on an annual basis It will combine the process for reporting ICOI and COIs in Research/IP Electronic System Network login information is used to log into ClickCommerce website Select the “New COI” button to access the electronic disclosure form Website: https://oric.utsa.edu Other Interests In addition to financial interests that must be managed, reduced, or eliminated, an Institution may require the management of other conflicting interests as the Institution deems appropriate. This includes managing Conflicts of Commitment to ensure that all employees carry out their duties to the University first, before commitments to any outside entity. Contact Information Bernard Arulanandam, Ph.D., MBA Chair, Committee on COI in Research & IP (210) 458-5492 bernard.arulanandam@utsa.edu Martha R. Treviño, M.A. Director, Ethical Conduct of Research Office of Research Integrity & Compliance (210) 458-4531 martha.trevino@utsa.edu Jennifer Morales Research Compliance Specialist Office of Research Integrity & Compliance (210) 458-4233 jennifer.morales@utsa.edu Website: http://vpr.utsa.edu/oric/coi