The Electric Force - Edvantage Science
advertisement

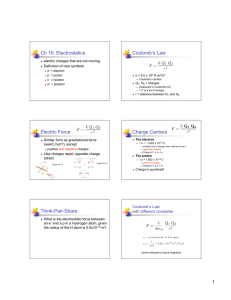
5.2 The Electric Force Coulomb’s Law Charles Coulomb (1736 – 1806) using an apparatus very much like the one Henry Cavendish used to measure the gravitational constant determined the relationship for the electric force between two charged objects. Coulomb found out that the force between two charges varied directly with each charge and inversely with the square of the distance between the charges. F = kQ1Q2 r2 Where Coulomb’s Constant: k = 8.988 x 109 N2m2/C2 p. 167 -168 5.2 The Electric Force The Coulomb and the Elementary Charge The charge on an electron or a proton is called the elementary charge. The elementary charge (determined by Robert Millikan) has a value of : e = 1.602 x 10-19 C And since 1 C of charge is the charge on 6.2422 x 1018 electrons: 1 1 e = 1.602 x 10-19 C = 6.2422 x 1018 C The elementary charge is just the reciprocal of the # of electrons in 1 Coulomb of charge. p. 168 5.2 The Electric Force The electric force like all forces are vector quantities. When determining the net electric force you need to always consider the direction of the force. Unlike gravitational force which has only one direction towards each mass, electric force can either be an attractive force or a repulsive force. The net force will the vector addition of all the electric forces acting on the charges. For example consider the net force on the right charge due to the other 2 charges. All three charges are identical. F1 F2 F1 = Fnet Fnet F2 p. 170 - 173 5.2 The Electric Force Key Questions In this section, you should understand how to solve the following key questions. Page 172 – Practice Problem 5.2.2: Page 175 - 176 – Review 5.2 #1,2,4,6,7, & 8