12 C 13 C - Vincent Sapone.Com
advertisement
Calculating Average Atomic Mass
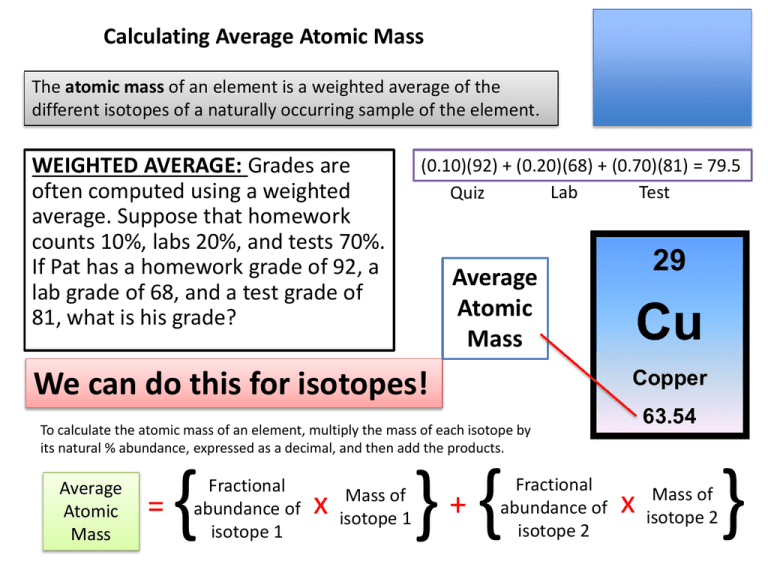
The atomic mass of an element is a weighted average of the
different isotopes of a naturally occurring sample of the element.
WEIGHTED AVERAGE: Grades are
often computed using a weighted
average. Suppose that homework
counts 10%, labs 20%, and tests 70%.
If Pat has a homework grade of 92, a
lab grade of 68, and a test grade of
81, what is his grade?
(0.10)(92) + (0.20)(68) + (0.70)(81) = 79.5
Lab
Test
Quiz
29
Average
Atomic
Mass
We can do this for isotopes!
Cu
Copper
63.54
To calculate the atomic mass of an element, multiply the mass of each isotope by
its natural % abundance, expressed as a decimal, and then add the products.
Average
Atomic
Mass
=
{
Fractional
abundance of
isotope 1
x
Mass of
isotope 1
} {
+
Fractional
abundance of
isotope 2
x
Mass of
isotope 2
}
Carbon-12
Carbon-13
6
If you had 100 Atoms of Carbon
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
12C
13C
Carbon
Carbon has an average atomic mass very close to
that of Carbon-12 because close to 99% of
naturally occurring carbon on earth is carbon-12
and only 1% is carbon-13.
12.011
Carbon has two stable isotopes,
Carbon-12 and Carbon-13. Calculate
the average atomic mass of carbon
using the data below.
12C
13C
Mass number/Isotope
Exact weight (amu)
Percent Abundance
Carbon-12
12.000000
98.90
Carbon-13
13.003355
1.10
(0.9890 x 12.000000) + (0.0110 x 13.003355) = 12.011amu
Carbon-12
Carbon-13
Fractional abundance is the percent abundance divided by 100!
Average
Atomic
Mass
=
{
Fractional
abundance of
isotope 1
x
Mass of
isotope 1
} {
+
Fractional
abundance of
isotope 2
x
Mass of
isotope 2
}
Nitrogen has two stable isotopes,
Nitrogen-14 and Nitrogen-15.
Calculate the average atomic mass
of nitrogen using the data below.
14N
15N
Mass number/Isotope
Exact weight (amu)
Percent Abundance
Nitrogen-14
14.003074
99.63
Nitrogen-15
15.000108
0.37
(0.9962 x 14.003074) + (0.0037 x 15.000108) = 14.007amu
Nitrogen-14
Nitrogen-15
Mass number vs. exact weight/atomic mass?
Average
Atomic
Mass
=
{
Fractional
abundance of
isotope 1
x
Mass of
isotope 1
} {
+
Fractional
abundance of
isotope 2
x
Mass of
isotope 2
}
amu
Average Atomic Mass of Magnesium?
% Abundance
What is the Fractional Abundance of Carbon-12 (12.00000 amu) and
Carbon-13 (13.003355 amu) granted that the average atomic mass of
Carbon is 12.011?
(12 amu * x ) + (13.003355 amu * y) = 12.011
But x + y must = 1 (must have 100%)
So writing y in terms of x yields
(12 amu * x ) + (13.003355 amu *(1- x)) = 12.011
Quirky Quantum Chemistry
• If a small marble has a mass of 3 grams and a large
marble has a mass of 5 grams how much will three
small marbles and two big marbles weigh in total?
• Well, atoms don’t follow these simple rules. Helium
has two protons and two neutrons but the mass of a
helium atom is actually less than the combined mass
of two protons and two neutrons. AN ATOM WEIGHS
LESS THAN THE SUM OF ITS PARTS!
• This is called a mass defect and its related to
Einstein’s equation E =mc2.
• Crudely: Some of the mass is converted into binding
energy used to hold the nucleons together.
1.
2.
3.
4.
5.
6.
7.
8.
What is the difference between the mass number and the exact atomic mass?
An atom cannot have a fraction of a neutron or a proton. Why is the exact mass of various elements not a
whole number? Hint, I am not asking about the weighted average on the periodic table?
The sum of its parts does not equal the whole. What does that mean in regards to an atom?
Boron has an atomic mass of 10.81 amu according to the periodic table. However, no single atom of boron
has a mass of 10.81 amu. How do you explain this?
The average atomic mass of Copper is 63.546amu. Which of copper’s two naturally occuring isotopes is more
common, Copper-63 or Copper-65?
Boron has two isotopes, Boron-10 and Boron-11. Which is more abundant given that the atomic mass of
Boron is 10.81.
Calculate the atomic mass of bromine. The two isotopes of bromine have atomic masses and relative
abundances of 78.92 amu (50.69%) and 80.92 amu (49.31%).
What is the % Abundance of Lithium-6 ( 6.015122 amu) and Lithium 7 (7.016004 amu) if the average atomic
mass of lithium is 6.94.
amu
% Abundance
9. Calculate the average atomic mass of Calcium.







