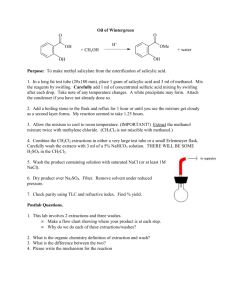
Chem. 31 – 9/15 Lecture
advertisement
Chem. 230 – 9/9 Lecture Announcements • Specialized Topics Presentation – I will have a sign up sheet available for next week – I would advise you to find a partner by next week • Next Tuesday: Guest Lecturer, Dr. Justin Miller-Schulze, on Solid Phase Extraction • List of Additional Resources Will be Posted on Website (some books, suggested journals and a website) Extractions – Liquid to Solid • Temperature in precipitation processes – Example: Acetic Acid and Water Liquid “path” for cooling 50% acetic acid in water Eutectic Point Liquid solution T Ice + CH3CO2H (l) CH3CO2H(s) + H2O(l) Solid solution 0 X(CH3CO2H) 100% Questions related to solid-liquid extractions 1. 2. 3. 4. What are advantages and disadvantages of Soxhlet extractions? Suggest a way to isolate a polar organic compound from ionic compounds in urine. It is desired to isolate CN- from CO32- by adding Ag+ and monitoring [Ag+] electrochemically. Assuming initial concentrations of [CN-] = 1.0 x 10-3 M and [CO32-] = 5.0 x 10-3 M and given Ksp values for AgCN and Ag2CO3 are 2.2 x 10-16 and 8.2 x 10-12, respectively, what would be the target [Ag+]? Will this separation be very efficient? Can acetic acid be isolated from all solutions in water by freezing it out? Additional Questions – cont. 5. 6. A 1.00 L sample of sea water is analyzed for phenols. The 1.00 L sample is passed through a solid phase extraction cartridge to trap the phenols. Then 25.0 mL of methanol is used to remove the phenols and then reagents are added that convert the phenols to methoxyphenols. The methoxyphenols are extracted by adding 25 mL of water to the methanol and extracting with two successive 25 mL portions of hexane. The hexane portions are combined, evaporated, and redissolved in 2.0 mL of hexane. An aliquot is then determined by GC and found to contain 22.1 mmol L-1 of a particular phenol. What was the original conc. of that phenol in sea water (in nmol L-1) if it is assumed that all transfers were 100% efficient? How could the sensitivity of the method be increased? The total NH3 (NH3 + NH4+) concentration of a water sample is determined by NH3 in the headspace above a sample. A water sample at a pH of 8.1 was found to have a headspace pressure of 2.4 x 10-7 atm. If KH = 62500 mol/atm m3 (at that T) and Ka(NH4+) = 5.6 x 10-10., calculate the total NH3 concentration in the sample Liquid-Liquid Extractions • One of more common simple separations • Often used to introduce partition theory • Equipment is simple (separation funnel or vials + syringes) • Two liquids must be immiscible (form two distinct phases) • Lower phase is more dense (usually water or chlorinated hydrocarbon) • Most common with water (or aqueous buffer) and less polar organic liquids Liquid-Liquid Extractions • Partition Coefficient Kp = [X]raffinate/[X]extractant • Kp depends on thermodynamics of dissolving X in two phases • Most common rule for solubility is likes dissolve likes • Water is typically one phase (but can use hexane/methanol) • More polar compounds exist in greater concentration in water • Koctanol-water values can be found in reference tables (octanol is assumed to be the raffinate) X(org) X(aq) If sample starts in aq phase, aq phase is raffinate, org is extractant Liquid-Liquid Extractions • Effect of organic solvent on Kp: – Small or large Kp values mean effective phase transfer – Less polar organic solvents (e.g. hexane) will give largest Kp values (assuming aqueous extractant phase) for non-polar analytes (e.g. octylstearate – CH3(CH2)7OC(O)(CH2)17CH3), but smaller values for analytes of modest polarity (e.g. a phenol) – For analytes of modest polarity, a more polar organic solvent will give a larger Kp value (e.g. ether or ethyl acetate) – This can be seen by using a “polarity” scale (note – a true “polarity” scale may be multidimensional) and looking at “difference” from solvent Polar H2O phenol ether octylstearate hexane Non-Polar Liquid-Liquid Extractions • Effect of organic solvent on Kp - Selectivity – When separating two analytes, finding a solvent with better selectivity also is important – Example: n-butanol and benzyl acetate (hexane vs. benzene as organic phase) – Ideally low Kp for analyte and high Kp for interferant – α = separation factor = (Kp)A/(Kp)B (where (Kp)A> (Kp)B) Liquid-Liquid Extractions • The fraction of moles extracted depends on both Kp and volumes of phases • k = partition ratio or retention factor (in chromatography) • For this example we will assume an organic raffinate • k = norg/naq • k = Kp(Vraf/Vext) = Kp(Vorg/Vaq) • Q = fraction extracted (to extractant or aq. phase) • Fraction left in raffinate = 1 - Q • Q = 1/(1 + k) Vorg X(org) X(aq) Vaq Liquid-Liquid Extractions Demonstrations • Methylene Blue (charged organic dye) – Which phase will it be in: hexane or water? • Iodine (I2 – non-polar – but polarizable compound) – Which phase will it be in: hexane or water? – Will Kp (organic extractant) get larger or smaller by changing from water to methanol? Or by changing hexane to ethyl acetate? Liquid-Liquid Extractions • To increase the fraction extracted, either Kp needs to be changed or the volume ratio • Example: If a compound is extracted from octanol to water and Kow = 0.8, how much volume of water is needed if the compound is in 10 mL of octanol and the extraction should move 90% of the compound? Liquid-Liquid Extractions • A different example, methylethylketone (MEK), CH3CH2COCH3, has Kow = 24, if 25 mL of aqueous MEK is extracted with 10 mL of octanol, calculate Kp, Q, and 1 – Q Liquid-Liquid Extractions • • Let’s look at multiple extractions in more detail MEK example Octanol – 91% MEK in 1st extraction to octanol transferred – 9% MEK left in water – In 2nd extraction of octanol (2nd water portion), fraction of original in water = Q(1 - Q) = 0.91*0.09 = 0.085, fraction of original in octanol = Q2 = 0.82 – In 2nd extraction of water portion by octanol, fraction in octanol = (1 – Q)Q = 0.085, fraction in water = (1 – Q)2 = 0.009 – If water is extracted twice with Fresh water added octanol and octanol fractions combined, fraction in octanol = 1 - (1 – Q)2 = 0.99 (99%) Octanol (extractant) Water (raffinate) Water transferred Fresh octanol added Liquid-Liquid Extractions • Efficiency of Multiple Extractions vs. Single Extractions • Back to Previous example (compound with Kow = 0.8 – before MEK) but using 3 successive 10 mL aliquots of water. – Note: successive extractions can be done in different ways (cross-current vs. counter current) – In this example (assuming only 2 components), the 10 mL water aliquots normally would be combined Liquid-Liquid Extractions Crown ether (12-crown-4) • Additional Equilibria – Crown ether example – Complexed metals become more organic soluble O O Na+ O Crown ether added Equilibria: 1) Na+(aq) + L(aq) ↔ NaL+(aq) 2) Sodium is not ether soluble O Diethyl ether 3) NaL+(aq) ↔ NaL+(ether) 4) L(aq) ↔ L(ether) Sodium conc. given by gray shading water Liquid-Liquid Extractions Additional Notes • Crown ethers and extractions • Extraction depends on: – Crown ether “hole” – Size of metal cation – Crown ether partition coefficients (rxn 3 and 4) Liquid-Liquid Extractions Other Methods for Transferring Metals • Metal – Ligand Complexes – Best transfer for neutral complexes with large organic ligands – Most common ligands are L- form and bidentate (2 bonds per ligand with metal) – Reactions: • • • • HL (aq) ↔ HL (org) HL (aq) ↔ H+ + LMn+ + nL- ↔ MLn (aq) (note this can be broken into n steps) MLn (aq) ↔ MLn (org) – Best at intermediate pH and for metals with large complexation formation constants • Ion – Pairs (e.g. Na+--O3S(CH2)5CH3) Liquid-Liquid Extractions Acidic/Basic Organics • Ions have very small Kow values (assume = 0) • Form of acids and bases in neutral species depends on pH • Distribution Coefficient = KD • KD = [X]total raffinate/[X]total extractant • Monoprotic acid example (HA extracted from organic to water) – HA = only organic phase species (a little different in text example) – HA and A- possible in aqueous phase Liquid-Liquid Extractions Acidic/Basic Organics HA example continued • Kp = [HA]org/[HA]aq = constant • KD = [HA]org/([HA]aq + [A-]) – not constant • Ka = [H+][A-]/[HA] • So KD = [HA]org/([HA]aq + Ka[HA]aq/[H+]) • KD = Kp/(1+ Ka/[H+]) – At pH << pKa (high [H+]), KD = Kp – At pH >> pKa, KD << Kp (better transfer to aq phase) Liquid-Liquid Extractions Acidic/Basic Organics • HA example KD = K p logKD Only HA present 0 HA and Apresent pKa pH 14 Liquid-Liquid Extractions Acidic/Basic Organics • In general, only un-ionized formed will be organic soluble (applies to multi-functional compounds also) • For weak bases, only base form B, not BH+, will be organic soluble • For weak bases, low pH increases partitioning into water Liquid-Liquid Extractions Some Questions • How can the following compounds be separated? O O O CH3 OH CH3 O OH O Kow = 15 pKa = 3.0 Kow = 40 pKa ~ 9.5 Kow = 130 CH3 Liquid-Liquid Extractions Some Questions KD sketch 1. Separate Comp. 1 in aq. phase from others at pH 7 2. Separate comp. 2 in aq. phase from comp. 3 at pH 13 3rd compound 2nd Compound logKD 1st Compound 0 3 pH 9 14 Liquid-Liquid Extractions Some Questions • To extract Al3+ to an organic phase with an HL type ligand, which complexation constant is most important? • Amino acids can act as bidentate ligands for metal transfer. Why does the pH have to be greater than pKa2? :NH2 M2+ CH2C6H5 -OC=O Liquid-Liquid Extractions One More Question • Benzylamine (C6H5CH2NH2) is a weak base with a Kow of 12. The conjugate acid of benzylamine has a pKa of 9.35. Benzylamine is being extracted from 10 mL of water buffered to a pH of 8.00 (raffinate phase) to octanol. (assume no other reactions occur in water or octanol) • Determine the distribution coefficient. • Calculate the fraction of benzylamine extracted into octanol if 20 mL of octanol is used? • How could Q be increased?