Lab-08-Alien
advertisement

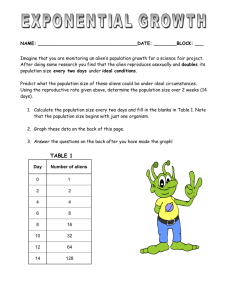
CHEMISTRY 2014-2015 SCHOOL YEAR CLASSROOM: 510 CODE: SCS21 INSTRUCTOR: Ms. Bui LAB ROOM: 506 LAB 08 ALIEN PERIODIC TABLE Name_______________________________________ Date: ______________________________________ Period: _____________________________________ Subject: Chemistry PRE-LAB: (________/6 points) Instructions: You have 3 minutes to complete this task. This is individual work. You are seated and silent. Look at the Periodic Table and complete the table below by identifying 3 ways (arrangement method) in which you think the Periodic Table is arranged. Make sure to include an example for each of the way. Arrangement Method Example Materials: Set of 40 Alien cards (85 sets) Periodic Table Lab Rubric Pre-Lab: _________/6 points Exploratory Activity: ____________/70 points Post-Lab: ___________/24 points Name_______________________________________ Date: ______________________________________ Period: _____________________________________ Subject: Chemistry EXPLORATORY ACTIVITY: ALIENS ARE HERE! We woke up to an amazing event today. An alien spaceship has landed in Brooklyn. The aliens seem friendly and want to establish relations with Earth. YOUR TASK: (______________/20 points) Our scientists want to have a better understanding of the aliens and need your help in organizing the aliens into an Alien Periodic Table. You will be working with a partner. This should take you about 10 minutes. 1. Arrange the aliens into group a. Every alien within a group must share one common feature (Key Similarity) b. Every alien within a group must share a feature that changes regularly as you move down the group (Varying Trait) 2. Arrange the aliens into periods a. Every alien within a period must share one common feature (Key Similarity) b. Every alien within a period must share a feature that changes regularly as you move across the period (from left to right) (Varying Trait) Once you have completed arranging your alien cards into an Alien Periodic Table, call Ms. Bui over. She will verify that you have the correct arrangement. Make sure you can provide REASONS on your arrangement. Questions: (__________/4 points) 1. How many groups of Aliens do you have? _____________ 2. How many periods of Aliens do you have? ____________ Ms. Bui’s Chemistry Class 2 Lab 08 Name_______________________________________ Date: ______________________________________ Period: _____________________________________ Subject: Chemistry Data Table: complete the following table by providing the key similarity and varying traits for each groups and periods (______________/26 points) Group or Period (circle one) group period group period group period group period group period group period group period group period group period group period group period group period group period Ms. Bui’s Chemistry Class Number Key Similarity 3 Varying Trait Lab 08 Name_______________________________________ Date: ______________________________________ Period: _____________________________________ Subject: Chemistry Drawing Activity: (_________/20 points) It has come to our attention that there are actually 2 more types of aliens on this planet. The first type belongs in the first group but is a new period. The second type belongs in the first period but is a new group. Instruction: List 5 traits describing each type of alien and provide a drawing Type 1 (First group, new period) Description: 1. Drawing 2. 3. 4. 5. Type 2 (First period, new group) Description: 1. Drawing 2. 3. 4. 5. Ms. Bui’s Chemistry Class 4 Lab 08 Name_______________________________________ Date: ______________________________________ Period: _____________________________________ Subject: Chemistry POST LAB (_________/24 points) 1. Compare the Alien Periodic Table to the Elemental Periodic Table. List three similarities. (9 pts) a. b. c. Before atomic numbers were known, Mendeleev developed a classification system for the 63 elements known in 1872, using oxide formulas and atomic masses. He used an R in the oxide formulas to represent any element in each group. The atomic mass was listed in parentheses after the symbol of each element. A modified version of Mendeleev's classification system is shown in the table below. 2. Identify two characteristics used by Mendeleev to develop his classification system of the elements (6 points). 3. Explain, in terms of chemical reactivity, whether or not Mendeleev would be able to include Group 18 (Noble Gas Group) in his version of the Periodic Table. State your claim and explain. (9 points) Ms. Bui’s Chemistry Class 5 Lab 08