To give light on the Earth
advertisement

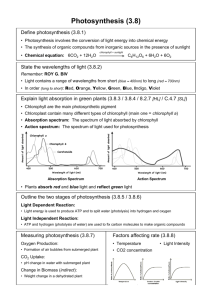
To Give Light on the Earth Genesis 1:15 Billions of Habitable Alien Planets Should Exist in Our Galaxy by Clara Moskowitz, SPACE.com Assistant Managing Editor Date: 28 March 2012 Extrapolating from Extrapolating from these findings, the researchers estimate that tens of billions of these planets are to be these findings, the found in the Milky Way, and about 100 should lie in the immediate neighborhood of the sun. (ibid) researchers estimate that tens of billions of these planets are to be found in the Milky Way, and about 100 should lie in the immediate neighborhood of the sun. (ibid) The Mediocrity Principle “The Earth is a mediocre (average) planet revolving around a very unremarkable star.” Hebrews 11:3 English Standard Version Anglicised 3 By faith we understand that the universe was created by the word of God, so that what is seen was not made out of things that are visible. "Now that we know that there are many super-Earths around nearby red dwarfs [a classification of star], we need to identify more of them using both HARPS and future instruments," said team member Xavier Delfosse. "Some of these planets are expected to pass in front of their parent star as they orbit — this will open up the exciting possibility of studying the planet's atmosphere and searching for signs of life." … the light coming from the star allows the components of the planet’s atmosphere to be analyzed. “To give light on the Earth” Genesis 1:15 THE ELEC ROMAGNETIC SPECTRUM The Visible Spectrum – the width of a tiny, tiny thread within the vast total spectrum Higher frequency wavelengths have higher energies – which are lethal to life. The Critical Protective Layers SOURCES OF GAMMA RADIATION • Radioactive elements here on Earth • Deep outer space sources – blocked by Earth’s atmosphere, no effect on life Marie Curie and her husband Pierre announced the discovery of radium on December 26, 1898. It was hailed as a ‘wonder drug.’ Radium Girls Radium Girls Coleman Lanterns Poisoned by Polonium: The Litvinenko File (2007) Wilhelm Rontgen 1895 Cancer of the fingers was an occupational disease common among dentists before the carcinogenic properties of x-rays were well understood. Dentists would hold the dental x-ray film in the mouths of patients while x-raying their teeth. (Source: ndt-ed.org) ULTRAVIOLET INFRARED DEMONSTRATIONS THE OZONE LAYER Levels of ozone at various altitudes and blocking of different bands of ultraviolet radiation. Essentially all UVC (100–280 nm) is blocked by dioxygen (from 100– 200 nm) or else by ozone (200–280 nm) in the atmosphere. The shorter portion of the UV-C band and the more energetic UV above this band causes the formation of the ozone layer, when single oxygen atoms produced by UV photolysis of dioxygen (below 240 nm) react with more dioxygen. THE OZONE LAYER … CONTINUED The ozone layer also blocks most, but not quite all, of the sunburn-producing UV-B (280–315 nm) band, which lies in the wavelengths longer than UV-C. The band of UV closest to visible light, UV-A (315–400 nm), is hardly affected by ozone, and most of it reaches the ground. UVA does not cause skin reddening, but there is evidence that it causes longterm skin damage. Solar radiation is electromagnetic radiation in the 280 nm to 3000 nm wavelength range. The solar spectrum includes a small share of ultraviolet radiation (280 nm to 380 nm) which is invisible to our eyes and comprises about 2% of the solar spectrum, the visible light which range from 380 nm to 780 nm and accounts for around 49% of the spectrum and finally of infrared radiation with long wavelength (780 to 3000 nm), which makes up most of the remaining 49% of the solar spectrum MECHANISMS OF OZONE FORMATION N2 absorbs radiation in the 80-100 nm range [Jnl Molecular Liquids 141 (2008 pp 110-117)] Ozone protects the DNA in all living things UV-B radiation can be harmful to the skin and is the main cause of sunburn; excessive exposure can also cause genetic damage, resulting in problems such as skin cancer. The ozone layer (which absorbs from about 200 nm to 310 nm with a maximal absorption at about 250 nm) is very effective at screening out UV-B; for radiation with a wavelength of 290 nm, the intensity at the top of the atmosphere is 350 million times stronger than at the Earth's surface. Nevertheless, some UV-B, particularly at its longest wavelengths, reaches the surface. DNA REPAIR MECHANISM CHILDREN WHO CANNOT PLAY IN THE SUN Oxygen (O2) is unstable in our planet’s atmosphere and must be constantly replenished by photosynthesis in green plants. Without life, our atmosphere would contain almost no O2. If we discover any other planets with atmospheres rich in oxygen, we will know that life is almost certainly present on these planets; significant quantities of O2 will only exist on planets when it is released by living things. (http://www.chemicool.com/elements/oxygen.html) THE SCARCITY OF OXYGEN O2 H2 O Free oxygen is too chemically reactive to appear on Earth without the photosynthetic action of living organisms, which use the energy of sunlight to produce elemental oxygen from water. (http://en.wikipedia.org/wi ki/Oxygen) REACTIVITY OF OXYGEN DEMONSTRATION The chlorophyll molecule is the only one in existence (perhaps possible) which can capture light energy and transfer that energy into a sophisticated energy processing system. Oxygen is the key to planetary life. No more than a waste product of photosynthesis, oxygen really is the molecule that makes a world. It is let loose by photosynthesis so fast that it finally overwhelms the capacity of a planet to swallow it up. In the end, all the dust and all the iron in the rocks, all the sulphur in the seas and methane in the air, anything that can be oxidised is oxidised, and free oxygen pours into the air and the oceans. (Nick Lane, Life Ascending, chapter 3) Many textbooks point out that our fossil fuels and carbohydrates derive their chemical energy from the sun. It should be noted that methane is abundant on many planets and moons far from the sun but free oxygen is found on none of them. There are plenty of oxygen atoms out there but they are all bound up with carbon, hydrogen, metals, silicon, and so forth. While the early “fuels” may result from the sun’s energy , it is really the oxygen molecules generated during photosynthesis that are trapping the energy of the sun and facilitating life on Earth. (Appreciating Oxygen, Hilton M. Weiss, Journal of Chemical Education, Volume 85, No. 9, September 2008, p. 1218) The gases O2 (and its photolytic product O3) N2O [nitrous oxide] and CH4 [methane] have been widely discussed in the literature as biosignature gases on planets with Earthlike composition atmospheres… O2 and its photolytic product O3 are the most reliable biosignature gas indicators for life as we know it. O2 is highly reactive and therefore will remain in significant quantities in the atmosphere only if it is continually produced. The major sink of O2 and O3 is surface reactions. There are no known abiological mechanisms that can continually produce large quantities of O2. (An Astrophysical View of Earth-based Metabolic Biosignature Gases, Sara Seager et al, Astrobiology, Volume 1, Number 1, 2012) Translation: Only life did and can generate the oxygen (and ozone) in the quantities we find in Earth’s atmosphere. June 29, 2012 The Daily Galaxy Mystery of The Missing Life-Giving Molecule in Space Deepens A new search for molecular oxygen in the Orion Nebula has come up negative, leading to new ideas on what's wrong in the chemical models. Searches for interstellar molecular oxygen, O2, have a long history… Further modeling and additional observations will clarify the situation further, but the present work goes a long way to narrowing the possible explanations for the mysterious absence of this life-giving molecule. Chlorophyll – the most important molecule on the Earth – can it be synthesized in the lab? PROFESSOR ROBERT WOODWARD Robert Burns Woodward 1917-1979 He received the Nobel prize in 1965 for Chemistry: “The synthesis of a complicated molecule is, however, a very difficult task; every group, every atom must be placed in its proper position and this should be taken in its most literal sense. It is sometimes said that organic synthesis is at the same time an exact science and a fine art. Here Nature is the uncontested master, but I dare say that the prizewinner of this year, Professor Woodward, is a good second.” METABOLIC PATHWAY OF PHOTOSYNTHESIS A General Metabolic Pathway of Living Organisms A Graphic Depiction of a Metabolic Pathway Quantum Effect Spotted in Early Stages of Photosynthesis (Science Daily June 2, 2012) Using ultrafast spectroscopy, the researchers observed the subatomic interactions at the initial stage of photosynthesis, which can take less than a trillionth of a second. To their surprise, they discovered a never-before-seen quantum effect whereby a single photon aroused different chromophores simultaneously. “The behavior we were able to see at these very fast time scales implies a much more sophisticated mixing of electronic states,” Tiede said. “It shows us that high-level biological systems could be tapped into very fundamental physics in a way that didn’t seem likely or even possible.” The quantum effects observed in the course of the experiment hint that the natural light-harvesting processes involved in photosynthesis may be more efficient than previously indicated by classical biophysics, said chemist Gary Wiederrecht of Argonne's Center for Nanoscale Materials. "It leaves us wondering: how did Mother Nature create this incredibly elegant solution?" he said. In summary, evolving oxygenic photosynthesis involves bringing together several major components, each of which has a number of potentially difficult steps in its evolution, and the overall scenario must somehow fit together in a way that each stage is is selected for (or at least not rapidly selected against.) Whilst some promising scenarios have been put forward, and nature may have offered a helping hand in assembling the water-splitting complex, we are still far from sure how it was done. Creating the whole looks formidably difficult, and far harder than evolving any of the forms of anoxygenic photosynthesis we see today. When faced with trying to explain how a complex and beautiful system such as oxygenic photosynthesis arose, the challenge for biologists is to show how it could have happened by plausible evolutionary steps, and we are still a long way from reaching this goal. (Revolutions that Made the Earth, Lenton & Watson, Oxford University Press, 2011, pp. 161-162) RESONANCE DEMONSTRATION Antennae are tuned to different frequencies Light Spectrum of the Sun Absorption Spectrum of Chlorophyll Light Spectrum of the Sun that reaches the Earth through the present atmosphere Absorption Spectrum of Chlorophyll Light Spectrum of the Sun that reaches the Earth through the present atmosphere Absorption Spectrum of Chlorophyll At this point there are two different types of chlorophyll (a and b for each of the two photosystems)… One type of chlorophylls called antenna chlorophyll; the second is the reaction center chlorophyll. The antenna chlorophyll collect light energy and transfer it (in about 1 trillionth of a second) to the reaction center, (National Science Foundation website) Star Light, Star Bright, Any Oxygen Tonight? Breathe deeply, and thank the nearest tree, bush, or blade of grass. The Earth receives most of its oxygen from photosynthetic activity. Breathe deeply, and thank the nearest tree, bush, or blade of grass. The Earth receives most of its oxygen from photosynthetic activity. The question of complex life on other worlds, therefore, is tied to the possibility of photosynthesis development. But could photosynthesis develop on worlds orbiting stars different from our sun? Star Light, Star Bright, Any Oxygen Tonight? The Great Oxidation Event O2 build-up in the Earth's atmosphere. Red and green lines represent the range of the estimates while time is measured in billions of years ago (Ga). Stage 1 (3.85–2.45 Ga): Practically no O2 in the atmosphere. Stage 2 (2.45–1.85 Ga): O2 produced, but absorbed in oceans & seabed rock. Stage 3 (1.85–0.85 Ga): O2 starts to gas out of the oceans, but is absorbed by land surfaces. Stages 4 & 5 (0.85–present): O2 sinks filled and the gas accumulates. The Great Oxygenation Event (GOE), also called the Oxygen Catastrophe or Oxygen Crisis or Great Oxidation, was the biologically induced appearance of free oxygen (O2) in Earth's atmosphere. This major environmental change happened around 2.4 billion years ago. Photosynthesis was producing oxygen both before and after the GOE. The difference was that before the GOE, organic matter and dissolved iron chemically captured any free oxygen. The GOE was the point when these minerals became saturated and could not capture any more oxygen. The excess free oxygen started to accumulate in the atmosphere. The Origin of Oxygen in Earth's Atmosphere The breathable air we enjoy today originated from tiny organisms, although the details remain lost in geologic time. It's hard to keep oxygen molecules around, despite the fact that it's the third-most abundant element in the universe, forged in the superhot, superdense core of stars. That's because oxygen wants to react; it can form compounds with nearly every other element on the periodic table. So how did Earth end up with an atmosphere made up of roughly 21 percent of the stuff? The answer is tiny organisms known as cyanobacteria, or blue-green algae. These microbes conduct photosynthesis: using sunshine, water and carbon dioxide to produce carbohydrates and, yes, oxygen. So a date and a culprit can be fixed for what scientists refer to as the Great Oxidation Event, but mysteries remain. What occurred 2.45 billion years ago that enabled cyanobacteria to take over? What were oxygen levels at that time? Why did it take another one billion years—dubbed the "boring billion" by scientists—for oxygen levels to rise high enough to enable the evolution of animals? Most important, how did the amount of atmospheric oxygen reach its present level? "It's not that easy why it should balance at 21 percent rather than 10 or 40 percent," notes geoscientist James Kasting of Pennsylvania State University. "We don't understand the modern oxygen control system that well." THE RISE OF OXYGEN Animals need oxygen. "You cannot evolve animals like us without having a significant amount of oxygen," says geochemist Dick Holland of Harvard University. "Without the Great Oxidation Event [a dramatic rise of oxygen in Earth's atmosphere some 2.3 billion years ago], we would not be here. No dinosaurs, no fish, no snakes - just a lot of microorganisms.“ (ASTROBIOLOGY MAGAZINE JULY 30, 2003) THE RISE OF OXYGEN (CONTINUED) Scientists are making progress on understanding the Great Oxidation Event, but still greater mysteries remain to be unraveled in the saga of oxygen on Earth. "Although we think we know when oxygen first appeared and rose, we know very little about its rise to the present level, especially about the relationship between atmospheric oxygen and the development of animals," says Catling There is evidence that oxygen levels also rose 1.3 billion years ago and again before the Cambrian Explosion, a rapid proliferation of animal life that began 540 million years ago. Some researchers believe increasing levels of atmospheric oxygen helped trigger the Cambrian Explosion. Catling says the reason for those rises in atmospheric oxygen "is even more of a mystery than the first one.“ "We want to understand what controls the rise of oxygen on the Earth and maybe other planets," says Kasting. "Oxygen is our best biomarker for looking for life on extrasolar planets." Metabolic pathways of Prochlorococcus marinus Prochlorococcus marinus lacks phycobilisomes that are characteristic of cyanobacteria, and contains chlorophyll b as its major accessory pigment. This enables it to absorb blue light efficiently at the low-light intensities and blue wavelengths characteristic of the deep euphotic zone. It contributes 30-80% of the total photosynthesis in the…oceans, and thus plays a significant role in the global carbon cycle and the Earth's climate. http://www.ebi.ac.uk/2can/genomes/bacteria/Prochlorococcus_marinus.html PROCHLOROCOCCUS MARINUS The strains which grow preferentially at depths between 80 and 200 meters form the low-light-adapted ecotype and contain a lot of divinyl-chlorophyll b, which absorbs optimally in the blue area of the spectrum. The genomes of these strains possess several copies http://www.genoscope.cns.fr/spip/Prochlorococcus-marinus-smallest.html Light intensity drops off dramatically in the depths of the oceans – blue light penetrates much further down due to the absorption spectra of water. Q: Could life exist here on Earth if the spectrum of the Sun was just every so slightly shifted toward the red end of the spectrum? THE IMPORTANCE OF CHLOROPHYLL B Plants produce 50% of the world’s oxygen CYANOBACTERIA Cyanobacteria (very, very tiny) – but numerically, among the most numerous organisms on Earth, contribute ~ 20% of the Earth’s free oxygen via photosynthesis Phytoplankton – single-celled diatoms (leave hard silica shells behind with remarkable geometrical shapes and colours) Large algae – includes seaweed Phytoplankton are microscopic ocean plants that form the base of ocean ecosystems; they are so abundant that they are visible from space. Here, average chlorophyll from 1998 through 2006 is shown in green and indicates areas of high biological productivity. Courtesy SeaWiFS Project/NASA GSFC and GeoEye, Inc. 9 Then God said, “Let the waters under the heavens be gathered into one place. Let the dry land be seen.” And it was so. 10 Then God called the dry land Earth. He called the gathering of the waters Seas. And God saw that it was good. 11 Then God said, “Let plants grow from the earth, plants that have seeds. Let fruit trees grow on the earth that bring their kind of fruit with their own seeds.” And it was so. 12 Plants grew out of the earth, giving their own kind of seeds. Trees grew with their fruit, and their kind of seeds. And God saw that it was good. 13 There was evening and there was morning, the third day. BILL NYE SAYS… 14 Then God said, “Let there be lights in the open space of the heavens to divide day from night. Let them tell the days and years and times of the year. 15 Let them be lights in the open space of the heavens to give light on the earth.” And it was so. 16 Then God made the two great lights, the brighter light to rule the day, and the smaller light to rule the night. He made the stars also. 17 God put them in the open space of the heavens to give light on the earth, 18 to rule the day and the night. He divided the light from the darkness. And God saw that it was good. 19 There was evening and there was morning, the fourth day. 20 Then God said, “Let the waters be full of living things. Let birds fly above the earth in the open space of the heavens.” 21 God made the big animals that live in the sea, and every living thing that moves through the waters by its kind, and every winged bird after its kind. And God saw that it was good. 22 God wanted good to come to them, saying, “Give birth to many. Grow in number. Fill the waters in the seas. Let birds grow in number on the earth.” 23 There was evening and there was morning, the fifth day. 24 Then God said, “Let the earth bring into being living things after their kind: Cattle and things that move upon the ground, and wild animals of the earth after their kind.” And it was so. 25 Then God made the wild animals of the earth after their kind, and the cattle after their kind, and every thing that moves upon the ground after its kind. And God saw that it was good. An Revelation 14:6-8 New Life Version (NLV) 6 Then I saw another angel flying in the heavens. He was carrying the Good News that lasts forever. He was preaching to every nation and to every family group and to the people of every language and to all the people of the earth. 7 He said with a loud voice, “Honor God with love and fear. The time has come for Him to judge all men. Worship Him Who made heaven and earth and the sea and the places where water comes out of the earth.” 1 Corinthians 3:18-20 J.B. Phillips New Testament (PHILLIPS) 18-19 Let no one be under any illusion over this. If any man among you thinks himself one of the world’s clever ones, let him discard his cleverness that he may learn to be truly wise. For this world’s cleverness is stupidity to God. It is written: ‘He catches the wise in their own craftiness’. 20 And again: ‘The Lord knows the thoughts of the wise, that they are futile’. Isaiah 45:18 For this is what the LORD says— he who created the heavens, he is God; he who fashioned and made the earth, he founded it; he did not create it to be empty, but formed it to be inhabited—he says: “I am the LORD, and there is no other.