Unit 8: Cell Respiration
advertisement

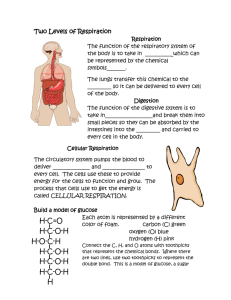
The process of using nutrients to release energy for use by the cell is called cellular respiration. In humans, cellular respiration is part of an elaborate metabolic process that allows us to maintain homeostasis. Homeostasis is the balanced internal environment of the body. The balanced equation for cellular respiration is: C₆H₁₂O₆ + 6 O₂ glucose oxygen consumption 6 CO₂ + 6 H₂O + 36 ATP carbon water energy dioxide production The rate of cellular respiration can be measured observing oxygen consumption or carbon dioxide production. 2 This experiment measures only the oxygen consumed by the mouse in this respiration chamber. 3 A mouse is weight and it’s mass recorded. Mass = 22.5 g 4 KOH (potassium hydroxide) pellets are put in the chamber to absorb the CO₂ produced by the mouse. Next, the cage with the mouse is placed in it. This is done so CO₂ production does not interfere with measuring of oxygen consumption. Pipette used to measure O₂ consumed Mouse in cage KOH pellets chamber 5 A bubble is placed at the end of the pipette, and measure the time passed for it to move a determined distance. This process is repeated 5 times. Bubble moving towards the mouse as it consumes the O₂ and the CO₂ it produces is absorbed by the KOH. 6 trial Volume of O2 used (ml) Time (min) Time (hours) (mlO2)/h (mlO2/h)/g 1 2 ml 3 3/60= 0.05 2/0.05= 40 40/22.5= 1.77 2 2 ml 3 0.05 40 1.77 3 2 ml 4 0.066 30.3 1.346 4 2 ml 2 0.033 60.6 2.693 5 2 ml 3 0.05 40 1.77 Average= 1.87 mlO2/h/g Organism: mouse Weight: 22.5 grams The number of minutes divided by 60 represents the number of minutes converted into hours. 7 Determine the relationship between body mass and metabolic rate. Animal (weight kg) Metabolic rate (mlO2/g/h) Kangaroo mouse (0.02kg) 1.8 Rat (0.10g) 0.87 Cat (10kg) 0.68 Dog (20kg) 0.33 Man (90kg) 0.21 Elephant (1,000kg) 0.07 Look at the chart above; as body mass increases, what happens to Decreases oxygen consumption? 8 Metabolic rate (VO2/h/g) Animal Size/weight (Kg) 9 Germinating seeds are living and growing plant embryos. The seeds of choice for this exercise are black-eyed peas. The seeds are soaked in water for a few minutes to start the germinating process. Experiment: Two beakers are used, each is added the strained, germinating seeds. The contents of only one beaker will be boiled in water for 5 minutes then drained and cooled. Germinated seeds Germinated–boiled seeds 10 Germinated seeds (Germ) are placed into one bottle and germinated-boiled seeds (Germ-Boil) are placed into a second bottle. Both bottles must contain roughly the same amount of seeds. 11 Stoppers are placed in the thistle tubes to prevent losing CO2 The respiration apparatus is assembled. The plastic tubing is placed into a test tube of water with phenol red. Phenol red is placed in the test tubes to trap any gas produced by the seeds. The bottles remain undisturbed for 40 minutes. Balanced equation: H₂O water + CO₂ H₂CO₃ carbon carbonic dioxide acid Phenol red is a pH indicator. It appears red at a neutral pH, and yellow at a low pH . 12 After the 40 minute incubation period, the gas in the bottle is displaced into the test tube by pouring tap water down the tube. Germinated seeds produced CO₂ lowering the pH shown by the color. Dead seeds did not respire, maintaining the color of neutral pH 13 Some eukaryotic cells will maintain their energy production by fermenting the products of glucose breakdown rather than respiring. One major eukaryotic fermentation produces ethanol + carbon dioxide. Alcoholic fermentation balanced equation: C₂H₁₂O₆ glucose 2 CO₂ + 2 C₂H₅OH + 2 ATP carbon ethanol energy dioxide 14 40 ml of solution (dH2O, glucose and sucrose) is placed in each of 3 beakers. Yeast is added to each of the 3 beakers and let sit for 5 minutes. 15 The mixture of yeast diluted in a solution are put in 3 different fermentation chambers (glucose 10%, Sucrose 10% anddH2O). Notice the markings of volume in ml Each chamber has a marking informing which solution it has. Fermentation chamber 16 The 3 samples are put in the incubator at 37°C and every 10 minutes the levels of carbon dioxide formed are read. The final production of CO₂ after 30 minutes looks like this. The yeast has settled, there are no bubbles here Water Notice the CO₂ bubbles sucrose 10% Notice the CO₂ bubbles overflow glucose 10% 17 Time 10% glucose 10% sucrose Water (0% glucose) 0 0 0 0 10 4.5 3 0 20 10 8 0 30 overflow 10 0 40 Once one of the chambers is overflown the experiment stops, usually by 20-30 minutes it is over. Sucrose is a disaccharide, but goes through a process that brakes it into fructose and glucose, and yeast is able to use those to produce ethanol. Look in the lab manual for details. The End 18