Poster on addiction
advertisement

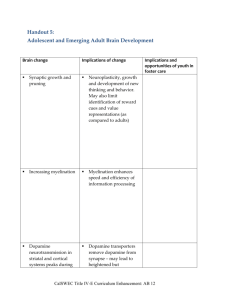
introduction Mesolimbic and mesocortical systems are involved with reward, planning and salience attribution. This research demonstrates different activity levels between controls and detoxified alcoholics in PFC control, striatal DA activity and subjective pleasure when challenged with the DAT blocker MP. This research demonstrates a correlation between prefrontal regulation of dopaminergic reward circuits in controls but not alcoholics. Subjective pleasure for drug liking and high in alcoholics are substantially reduced Mesolimbic circuits adapt to long term addiction including alcoholism. This research demonstrates profound reductions in DA receptor availability in detoxified alcoholics. Addiction theory • Natural reinforcement: • Reward for natural reinforcers such as food and water are mediated through the mesolimbic system. • Many addictive drugs including alcohol use the same reward system1 • DA changes in VS are correlated with insula metabolism. Insula region associated with densest DA innervation7 and addiction • Tolerance: • Profound reductions in DA activity in VTA is observed on withdrawal from addictive drugs including chronic alcohol abuse10 • Addiction reduces availability of DA receptors in VS10. Baseline striatal DA receptor availability associated with PFC metabolism in drug abuse8a,8b. Genetic risks8c • Craving: • Reduced receptors in VS associated with increased craving and greater incentive attribution and activation of medial PFC and CG11. • Pleasure vs. reward • Disruption to OFC, involved in salience attribution11 and/or the CG, involved with inhibitory control6 considered central to addiction • The PFC is associated in attribution salience and the addiction process schematic INCENTIVE SALIENCE PFC/OFC DA Loss of control in addicts glut Striatum Ventral striatum NAc DA VTA reward Putamen Caudate Less DA IN ADDICTS method • Methylphenidate (MP) challenge • Subjective behavioural effects • PET: Glucose metabolism in PFC • (ROI’s OFC, CG, DLPFC) • PET: Dopamine release in Striatum • (ROI’s CDT, PUT, VS) • 20 controls & 20 detoxified alcoholics • Single blind crossover design results D2/D3 receptor availability in VS Regression OFC:DA addictso controls• 7 Subjective liking and high 6 3.5 5 3 2.5 4 2 3 1.5 addicts 1 control s addicts 2 1 0.5 0 PL MP 0 0 2 4 6 discussion • Experimental hypotheses: in alcoholics - decreased DA activity; disrupted regulation of PFC; less subjective pleasure. • Results show: • In controls OFC activity negatively correlated with MP induced DA changes in NAc is consistent3,4 with OFC regulation of NAc via VTA glutamatergic efferents5 • In alcoholics vs. controls decreased DA release in VS consistent with other drugs of abuse10 • In alcoholics reduced subjective reward as measured by drug high(-70%) and liking(-50%) is consistent with drug tolerance • In alcoholics lower baseline receptor availability is consistent with adaptive changes to drugs of abuse and to genetic risk factors2,8 • In alcoholics lower receptor availability is associated with activity in PFC (CG, DLPFC) consistent with other drugs of abuse Conclusions • Loss of OFC modulation in mesolimbic systems of alcoholics and reduced DA increases in VS consistent with reduced reward systems activation • Reduced DA cell activity in reward systems consistent with reduced subjective pleasure in alcoholics • Reduced rewards and pleasure could lead to increased alcohol consumption • Therapy should address both the profound reduction in DA activity and loss of PFC modulation • Exploratory confirmation of correlation between DA changes in VS and insula metabolism Future directions: complex theory Integrated systems To be integrated •DA/NAc reward, +ve reinforcement system1 •Adaptive changes to DA circuits in addiction2,10 •DA decreases in striatum for addicts(cocaine, alcohol) 10 •PFC loss of control of VTA in addiction3 •(PFC hypoactive in cocaine addicts9) •(Genetic factors associated with reduced DA receptor activity8c) • Opiods needed for reinforcement, opiate receptors sufficient14 • PFC-grey reduction of 10% in alcoholics12 • Psychosocial stress associated with D2 reduced receptor activity and predilection for addiction13 • Incentive salience11 References • • • • • • • • • • • • • • • • • • • • Student: B0239046 John P Jeffrey 1 Koob GF, Roberts AJ, Schulteis G, Parsons LH, Heyser CJ, Hyytia P, Merlo-Pich E, Weiss F (1998) Neurocircuitry targets in ethanol reward and dependence. Alcohol Clin. Exp Res 22:3–9. 2a) Robbins TW, Everitt BJ (2002) Limbic-striatal memory systems and drug addiction. Neurobiol Learn Mem 78:625– 636. 2b )Nestler EJ (2004) Molecular mechanisms of drug addiction. Neuropharmacology 47 [Suppl 1]:24 –32. 3a) White FJ, Hu XT, Zhang XF, Wolf ME (1995) Repeated administration of cocaine or amphetamine alters neuronal responses to glutamate in the mesoaccumbens dopamine system. J Pharmacol Exp Ther 273:445– 454. 3b) Kalivas PW (2004) Glutamate systems in cocaine addiction. Curr Opin Pharmacol 4:23–29. 4a) Gariano RF, Groves PM (1988) Burst firing induced in midbrain dopamine neurons by stimulation of the medial prefrontal and anterior cingulate cortices. Brain Res 462:194 –198. 4b) Murase S, Grenhoff J, Chouvet G, Gonon FG, Svensson TH (1993) Prefrontal cortex regulates burst firing and transmitter release in rat mesolimbic dopamine neurons studied in vivo. Neurosci Lett 157:53–56 5 Kalivas PW, Volkow ND (2005) The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry 162:1403–1413. 6 Volkow ND, Fowler JS, Wang GJ (2003) The addicted human brain: insights from imaging studies. J Clin Invest 111:1444 –1451 7 Gaspar P, Berger B, Febvret A, Vigny A, Henry JP (1989) Catecholamine innervation of the human cerebral cortex as revealed by comparative immunohistochemistry of tyrosine hydroxylase and dopamine-betahydroxylase. J Comp Neurol 279:249 –271. 8a) Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, Dewey SL, Wolf AP (1993b) Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse 14:169 –177. 8b) Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N (2001) Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry 158:2015–2021. 8c) Volkow ND, Wang G-J, Begleiter H, Porjesz B, Fowler JS, Telang F, Wong C, Ma Y, Logan J, Goldstein R, Alexoff D, Thanos PK (2006) High levels of dopamine D2 receptors in unaffected members of alcoholic families: possible protective factors. Arch Gen Psychiatry 63:999 –1008. 9 Volkow et al.,(1992) cited by Carlson pp. 620 10 Volkow et al.,(2001) cited by Carlson pp.628 11 Heinz A, Siessmeier T, Wrase J, Hermann D, Klein S, Grusser SM, Flor H, raus DF, Buchholz HG, Grunder G et al (2004) Correlation between dopamine D(2) receptors in the ventral striatum and central processing of alcohol cues and craving. Am J Psychiatry 161:1783–1789. 12 Mathalon et al.,(2003) cited by Carlson pp.621 13 Morgan et al.,(2002) cited by Carlson pp.622 14 Carlson “effects of naloxone” pp.626: Carlson, N. R., (2007), ‘Physiology of Behaviour’, 9th Edition’, Pearson International, USA Reduced reward in detoxified alcoholics Loss of control by prefrontal cortex