Carbohydrates
advertisement

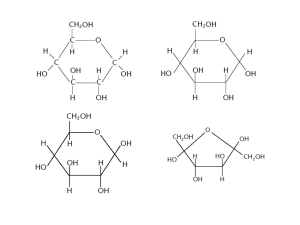
All B3 Objectives B.3.1: Describe the structural features of monosaccharides. B.3.2: Draw the straight-chain and ring structural formulas of glucose and fructose. B.3.3: Describe the condensation of monosaccharides to form disaccharides and polysaccharides. B.3.4: List the major functions of carbohydrates in the human body. B.3.5: Compare the structural properties of starch and cellulose, and explain why humans can digest starch but not cellulose. B.3.6: State what is meant by the term dietary fibre. B.3.7: Describe the importance of a diet high in dietary fibre. Objective B.3.1 Describe the structural features of monosaccharides • Monosaccharides have an empirical formula of CH2O – Many isomers • Ex: Glucose and fructose have same molecular formula but have different structures Glucose Objective B.3.1 • • • • Describe the structural features of monosaccharides Made up of covalent bonds Contain one carbonyl group (C=O) Contain at least two hydroxyl groups (-OH) Ex. Glucose, fructose, and galactose Objective B.3.2 Draw the straight-chain and ring structural formulas of glucose and fructose Glucose: Straight-Chain Formula • 6-carbon backbone • Carbons 1 and 5 are connected by Oxygen • Each carbon is bonded to a hydroxide (except for the 5th) – Carbon 1 has a hydroxide on top – Carbon 3 has a hydroxide on the left – Carbons 2, 4, and 6 have hydroxides on the right • All other bonds are occupied with Hydrogen Objective B.3.2 Draw the straight-chain and ring structural formulas of glucose and fructose Glucose: Ring-Structure Formula • Should be familiar (biology class) • Hexagon-shape with 5 Carbons and Oxygen in the top right corner • Each carbon bound to 1 hydrogen and 1 hydroxide – Hydrogen is on top, hydroxide is on bottom (except Carbon 3) – Carbon 5 has another carbon(6) instead of a hydroxide – Carbon 6 has 2 hydrogens and 1 hydroxide The ring-structure forms when glucose is dissolved in water and undergoes and internal reaction Objective B.3.2 Draw the straight-chain and ring structural formulas of glucose and fructose Alpha (α) vs. Beta (β) •Alpha (α) structure has the hydroxide group on the bottom bonded with carbon 1 in the ring structure. (AB) •Beta (β) structure has the OH group on the top bonded to carbon 1 in ring structure. (BT) •The alpha- and beta- variations only occur in the ring structure Objective B.3.2 Draw the straight-chain and ring structural formulas of glucose and fructose Fructose: Straight-Chain Formula • 6-carbon backbone • Carbon 1 and Carbon 6 both O H have two hydrogens and 1 C hydroxide H C OH • Carbon 2 has one single HO C H Oxygen H C OH • Carbons 3, 4, and 5 all have H C OH one hydrogen, one hydroxide CH2OH – Carbon 3 is flipped glucose (hydroxide on the left) O CH2OH C HO C H H C OH H C OH CH2OH fructose Objective B.3.2 Draw the straight-chain and ring structural formulas of glucose and fructose Fructose: Ring-Structure Formula • Pentagon-shape with Oxygen at the top center and 4 other Carbons • Two bottom Carbons have one hydrogen and one hydroxide • The two side Carbons have one hydroxide and another carbon • Hanging carbons have two The ring-structure forms when hydrogens and one fructose is dissolved in water and undergoes and internal hydroxide reaction Objective B.3.2 Draw the straight-chain and ring structural formulas of glucose and fructose Alpha (α) vs. Beta (β) •Similar to the Alpha and Beta structures of glucose •ARB (Alpha (α): on the right of C2 for straight-chain, on the bottom for ring structure) •BLT (Beta (β): on the left of C2 for straight-chain, on the top for ring structure) •Note that the fructose variations occur at C2, and not C1. Objective B.3.3 Describe the condensation of monosaccharides to form disaccharides and polysaccharides. • Monosaccharides can join together and form a disaccharide through condensation (dehydration synthesis) • Hydroxyl (-OH) groups of monosaccharides (or disaccharides) • Maltose Example (glucose + glucose) – C6H12O6 + C6H12O6 C12H22O11 + H2O – Hydrogen from one glucose –OH group and OH from another glucose’s –OH group are lost as water – Remaining oxygen up bridges the monomers forming a disaccharide – 14 linkage utilizing covalent bond (glycosidic bond) Objective B.3.3 Describe the condensation of monosaccharides to form disaccharides and polysaccharides. H H glucose glucose + H2O maltose Objective B.3.3 Describe the condensation of monosaccharides to form disaccharides and polysaccharides. • Monosaccharide examples: – Glucose, Fructose, Galactose • Disaccharide examples: – Lactose, Maltose, Sucrose • Polysaccharide examples: – Starch, Glycogen, Cellulose Objective B.3.3 Describe the condensation of monosaccharides to form disaccharides and polysaccharides. • Disaccharide Structures: – Lactose (in milk) • 1 beta-glucose + 1 beta-galactose – Maltose (starch digestion product) • 2 alpha-glucose – Sucrose (canesugar, common in food) • 1 alpha-glucose + 1 beta-fructose Sucrose Objective B.3.4 List the major functions of carbohydrates in the human body • Major functions: –Energy Source –Energy Storage –Important for Other Molecules Objective B.3.4 List the major functions of carbohydrates in the human body • Energy Source: Glucose –Glucose is a monosaccharide which helps provide the body with energy –Glucose is oxidized in respiration to help form ATP energy for the body to use http://www.individualsole.com/wp-content/uploads/2010/05/SpSu10_Running_02035_ipod-540x360.jpg Objective B.3.4 List the major functions of carbohydrates in the human body http://drpinna.com/wp-content/uploads/2010/08/glucose.gif Objective B.3.4 List the major functions of carbohydrates in the human body • Energy Storage: Glycogen – Energy, in animals, is stored in the form of Glycogen (in the liver muscles) – Glycogen is formed through Glycogenesis when there’s an abundance of glucose in the body – The polysaccharide Glycogen breaks down through Glycogenolysis when more energy is needed http://findstorageauctionriches.com/IMAGES/self-storage-units.jpg Objective B.3.4 List the major functions of carbohydrates in the human body http://themedicalbiochemistrypage.org/images/glycogen.jpg Objective B.3.4 List the major functions of carbohydrates in the human body • Carbs are Important for Other Molecules! – Carbs can be precursors to the formation of other molecules – EX. Glucose – Glucose is needed to produce Vitamin C , proteins, and in forming disaccharides and polysaccharides – In Glycolysis, glucose undergoes phosphorylation which allows it to be a precursor – Carbs are also involved in structure/support in plants especially (EX. Cellulose which is formed from glucose) Ascorbic Acid (Vitamin C) http://upload.wikimedia.org/wikipedia/commons/8/81/Ascorbic_acid_structure.png Objective B.3.5 Compare the structural properties of starch and cellulose, and explain why humans can digest starch but not cellulose. •Cellulose and Starch are both polymers of glucose •The ring structure of glucose has two orientations • α- Glucose •OH group on the carbon 1 and the CH2OH group on the carbon 5 point in opposite directions •β - Glucose • OH group and CH2OH group point in the same direction Objective B.3.5 Compare the structural properties of starch and cellulose, and explain why humans can digest starch but not cellulose. – Starch • Polysaccharide – Created with a chain α- Glucose units – Bridging O atom is on the opposite side of the CH2OH group • Serves as food storage in plants – Corn, potatoes, wheat, and rice contain starch • Two forms of Starch – Amylose » Straight chain polymer between the 1,4 carbons of the α- Glucose units (unbranched) – Amylopectin » Branched structure that has both α- 1,4 linkage and α- 1,6 linkage – The two forms of starch allow it to be a relatively compact spiral structure stored as starch grains in plant cells. Objective B.3.5 • Compare the structural properties of starch and cellulose, and explain why humans can digest starch but not cellulose. Cellulose – Polysachharide – Created with a unbranched chain β - Glucose units – Bridging O atom is on the same side as the CH2OH group – Β- 1,4 linkage – forms uncoiled linear chains due to the “upside down” alternating glucoses – Hydrogen Bonds – These form cables known as microfibrils which are rigid and give support to plants and make wood a useful building material Objective B.3.5 • • Compare the structural properties of starch and cellulose, and explain why humans can digest starch but not cellulose. Enzymes that break down starch cannot always break down cellulose because of their structural differences In humans, starch can be hydrolyzed to glucose and oxidized into energy – Cellulose passes through the body unchanged – Some animals and bacteria contain enzymes to digest cellulose as a food source – Cellulase breaks down the beta glycosidic bonds. Humans do not produce this enzyme Objective B.3.6 State what is meant by the term dietary fibre. • Dietary fibre is mainly plant material – Characteristics: • Can’t be hydrolysed (digested) by enzymes in the human digestive tract • may be digested by microflora in the gut • Examples: – Cellulose – Hemicellulose – Lignin – Pectin Objective B.3.7 Describe the importance of a diet high in dietary fiber. • Dietary fiber passes through the body without being changed or digested much. • Aids the health of the large intestine by stimulating the production of mucous and helping the other products of digestion to pass out of the body more easily. • Foods that are high in fiber: bran, dried herbs, spices, and peppers, soy beans, dark chocolate, and nuts. • Prevents: – – – – – Constipation Obesity Crohn's disease Hemorrhoids Diabetes mellitus References • http://www.elmhurst.edu/~chm/vchembook/540carbohy drates.html • http://ibchem.com/IB/ibnotes/brief/pdf/optB.pdf • http://www.chem.purdue.edu/courses/chm333/Fall%202 009/Lectures/Fall%202009%20Lecture%2028.pdf • http://www.mansfield.ohiostate.edu/~sabedon/biol1025.htm • http://www.3dchem.com/molecules.asp?ID=423 • http://www.edinformatics.com/math_science/science_of _cooking/glucose.htm • http://www.elmhurst.edu/~chm/vchembook/547cellulos e.html