Standards of Measurement
advertisement

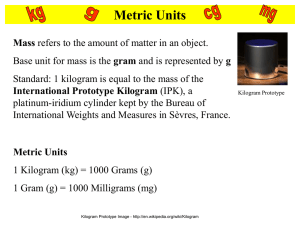
Standards of Measurement Standards Covered PS-1.3 Use scientific instruments to record measurement data in appropriate metric units that reflect the precision and accuracy of each particular instrument PS-1.5 Organize and interpret data from a controlled scientific investigation by using mathematics (including formulas and dimensional analysis), graphs, models, and/or technology Units and Standards • Standard – an exact quantity that people agree to use for comparison to represent a measurement or some other quality • Le Systeme Internationale d’Unites, SI System, is the system used by scientists and the rest of the world to make measurements – Each type of measurement has a base unit – Each base unit has a standard – Prefixes used with base units that are based on multiples of ten Length Metric Units Length: The distance from point to point. The basic unit of length in the SI system is the meter and is represented by a lowercase m. Standard: The distance traveled by light in absolute vacuum in 1⁄299,792,458 of a second. Measured using a metric ruler or a meter stick. Metric Units 1 Kilometer (km) = 1000 meters 1 Meter = 100 Centimeters (cm) 1 Meter = 1000 Millimeters (mm) Which is larger? A. 1 meter or 105 centimeters C. 12 centimeters or 102 millimeters B. 4 kilometers or 4400 meters D. 1200 millimeters or 1 meter English vs. Metric Units Which is longer? A. 1 mile or 1 kilometer B. 1 yard or 1 meter 1 mile 1.6 kilometers C. 1 inch or 1 centimeter 1 inch = 2.54 centimeters 1 yard = 0.9444 meters Left Image: http://webapps.lsa.umich.edu/physics/demolab/controls/imagedemosm.aspx?picid=1167 Right Image: http://share.lancealan.com/N800%20ruler.jpg How to Read a Metric Ruler The large lines are the cm The small lines in between are the millimeters - Notice there are 10 mm in 1 cm 1) Line up one edge of what you are measuring, with the zero mark on the ruler 2) Read all the known digits in the measurement, then estimate ONE place value past the known digits… ‘Tell what you know… then estimate one number further.’ Measuring Length How many millimeters are in 1 centimeter? 1 centimeter = 10 millimeters 2.80 What is the length of the line in centimeters? _______cm 28.0 What is the length of the line in millimeters? _______mm Ruler: http://www.k12math.com/math-concepts/measurement/ruler-cm.jpg A 43.5 mm 4.35 cm 60.6 mm 6.06 cm B C 8.5 mm 0.85 cm 30.0 mm 3.00 cm D Volume Metric Units Volume is the amount of space an object takes up. The base unit of volume in the metric system is the liter (L or l) for liquids and cubic centimeter (cm3) for solid objects. Standard: 1 liter is equal to one cubic decimeter Metric Units 1 liter (L) = 1000 milliliters (mL) 1 milliliter (mL) = 1 cm3 (or cc) = 1 gram* Measured using a Graduated Cylinder Which is larger? A. 1 liter or 1500 milliliters B. 200 milliliters or 1.2 liters C. 12 cm3 or 1.2 milliliters* * When referring to water Liter Image: http://www.dmturner.org/Teacher/Pictures/liter.gif English vs. Metric Units Which is larger? 1 fl oz = 29.573 ml A. 1 liter or 1 gallon 1 12-oz can of soda would equal approximately 355 ml. B. 1 liter or 1 quart C. 1 milliliter or 1 fluid ounce 1 quart = 0.946 liters 1 gallon = 3.79 liters It would take approximately 3 ¾ 1-liter bottles to equal a gallon. Measuring Volume We will be using graduated cylinders to find the volume of liquids and other objects. Read the measurement based on the bottom of the meniscus or curve. When using a real cylinder, make sure you are eye-level with the level of the water. What is the volume of water in the cylinder? _____mL 43.0 What causes the meniscus? A concave meniscus occurs when the molecules of the liquid attract those of the container. The glass attracts the water on the sides. Top Image: http://www.tea.state.tx.us/student.assessment/resources/online/2006/grade8/science/images/20graphicaa.gif Bottom Image: http://morrisonlabs.com/meniscus.htm What is the volume of water in each cylinder? 37.0 mL 52.0 mL 23.0 mL Pay attention to the scales for each cylinder. Images created at http://www.standards.dfes.gov.uk/primaryframework/downloads/SWF/measuring_cylinder.swf Measuring Liquid Volume Measuring Solid Volume 9 cm We can measure the volume of a regular object using the formula: length x width x height. 8 cm _____ _____ 10cm X _____ 8cm X _____ 9cm = 720 cm3 10 cm We can measure the volume of irregular object using: water displacement. mL Amount of H2O with object =260 ______ mL About of H2O without object =200 ______ 60 mL =60 cm3 Difference = Volume = ______ ______ Mass Metric Units Mass refers to the amount of matter in an object. The base unit of mass in the metric system in the kilogram and is represented by kg. Standard: 1 kilogram is equal to the mass of the International Prototype Kilogram (IPK), a platinumiridium cylinder kept by the BIPM at Sèvres, France. Mass is measured using a triple beam balance. Kilogram Prototype Metric Units 1 Kilogram (km) = 1000 Grams (g) 1 Gram (g) = 1000 Milligrams (mg) Which is larger? A. 1 kilogram or 1500 grams C. 12 milligrams or 12 kilograms B. 1200 milligrams or 1 gram D. 4 kilograms or 4500 grams Kilogram Prototype Image - http://en.wikipedia.org/wiki/Kilogram English vs. Metric Units Which is larger? 1. 1 Pound or 100 Grams 1 pound = 453.6 grams 2. 1 Kilogram or 1 Pound 3. 1 Ounce or 1000 Milligrams 1 ounce of gold = 28,349.5 milligrams 100 kilogram = 220 pounds Measuring Mass We will be using triple-beam balances to find the mass of various objects. To begin, you must ‘calibrate’ the balance. The ‘weights’ are all aligned to the far left… near the tray… then you turn the knob under the tray until you get the lines on the right-side of the scale to match up. Once you have calibrated the balance and placed the ‘tares in their notches’, you add up the amounts on each beam to find the total mass. What would be the mass of the object measured in the picture? 373.35 g 70 + _______ 3.35 = ________ 300 + ______ _______ Top Image: http://www.southwestscales.com/Ohaus_Triple_Beam_750-SO.jpg Bottom Image: http://www.regentsprep.org/Regents/biology/units/laboratory/graphics/triplebeambalance.jpg Measuring Mass – Triple-Beam Balance 1st – Place the object on the balance, in the center of the tray. 2nd – Slide the large weight to the right until the arm drops below the line. Move the tare back one notch. Make sure it ‘locks’ into place. 3rd – Repeat this process with the top weight. When the arm moves below the line, back it up one notch. 4th – Slide the small slider tare on the front beam until the lines match up. 5th – Add the amounts on each beam to find the total mass to the nearest tenth of a gram, then estimate one number further. 137.45g 203.25g 43.05g Time and Temperature Time - Interval between two events The base unit of time is the second (s). Standard: The frequency of the cesium-133 atom as the ‘reference clock’ Time is measured with a clock/ stop watch. Temperature – “how hot or cold something is” The base unit in the ‘old’ Metric system is degrees Celsius (˚C) The base unit in the modern SI system is the Kelvin (K) Standard: Based on freezing and boiling points of pure water at standard temperature and pressure… (0˚C and 100˚C at 1atm) Measured with a thermometer °C + 273 = K Thirty is HOT, Twenty is NICE, Ten is CHILLY, and Zero is ICE!! Accuracy and Precision Scientific Data • Accuracy – How close value is to accepted value (control) • Precision – how close repeated measurements are to one another – Determined by the measuring device being used – The smaller the Graduations… the more Precise the measurement! And the more likely it is to be repeated SI Prefixes Kilo (k) = 1000x hecta (h) = 100x deka (dk) = 10x deci (d) = .1x or 1/10 centi (c) = .01x or 1/100 milli (m) = .001x or 1/1000 Standards Covered PS- 1.2 Use appropriate laboratory apparatuses, technology, and techniques safely and accurately when conducting a scientific investigation. PS- 1.9 Use appropriate safety procedures when conducting investigations. A Special Relationship for Water 1 milliliter (ml) = 1 cubic centimeter (cm3) = 1 gram (g) For Everything Else 1 milliliter (ml) = 1 cubic centimeter (cm3) Solids - cm3 Liquids – ml Gases – either one DON’T FORGET YOUR UNITS!! Measurement Lab