Chapter1ScienceSkills9-13
advertisement

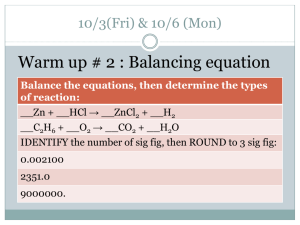
Chapter 1 Science Skills Page 2 1.1 What is science? (3:54) Science From Curiosity • Science - system of knowledge & methods used to find it • Begins w/ curiosity…ends w/ discovery • Curiosity provides ?’s • Observing/measuring…means to find answers Science and Technology Science and Technology (1:39) • Science / technology interdependent – Advances in 1 leads to advances in the other Branches of Science Natural Science Physical Science •Chemistry •Physics Earth and Space Science •Geology •Astronomy •Life Science •Biology Big Ideas of Physical Science • Space and Time – universe age – size • Matter and Change – Small amt for universe – Volume/mass – Atoms • Protons, neutrons, electrons • Forces and Motion – Push/pull causes change – Laws will explain • Energy – Many forms – Drives motion – Transferred/never destroyed Sec 1.2 Using a Scientific Approach p. 7 • Scientific Method - organized plan for gathering, organizing, & communicating info – Goal….to solve problem or better understand observed event Copy flow chart into your notes A Scientific Method • Making Observations – Observation – info that you obtain through your senses – Inference – conclusions drawn based on observations • Develop ? / problem • Form Hypothesis – proposed answer to ? – testable • Testing a hypothesis – Manipulated (independent) variable – changed by you to test hyp. – Responding (dependent) variable – changes in response to man. var. – Controlled variable – factors kept constant to test hyp. – Control Group –setup run w/o man.var. • Draw Conclusions – does data support hyp? • Develop Theory – Scientific Theory – well-tested explanation for observations or experimental results • Tells “why” • Never “proved” • May be revised/replaced Scientific Method Rap Mr. Duey Scientific Laws • Scientific law – summarizes pattern found in nature • explains “what” • DOES NOT attempt to explain observed pattern in nature Scientific Models • Scientific Models - makes easier to understand things too hard to observe directly – Ex. Atomic models, models of the solar system, cell models, etc. The Scientific Method (12:07) Section 3 Measurements p. 14 Using Scientific Notation Written as # btwn 1 & 10 and a power of 10 Makes very large/small #’s easier to work w/ The Distance From the Sun to the Earth 93,000,000 miles Step 1 • Move decimal left • Leave only 1 # in front of decimal Step 2 • Write the # w/o zeros Step 3 • Count # places decimal moved • That’s power of ten Standard Form Scientific Notation Practice Problem Write in scientific notation. Decide the power of ten. 1) 2) 3) 4) 98,500,000 = 9.85 x 10? 64,100,000,000 = 6.41 x 10? 0.0000000279 = 2.79 x 10? 0.00042 = 4.2 x 10? 9.85 x 107 6.41 x 1010 2.79 x 10-8 4.2 x 10-4 Complete Practice Problems Write in scientific notation. 4 10 5x 1) 50,000 6 7.2 x 10 2) 7,200,000 3) 802,000,000,000 8.02 x 1011 Multiplying numbers in scientific notation • When x #s in sci not, its 2 steps: A rectangular parking lot has a 3 length of 1.1 x 10 meters and width of 2.4 x 103 meters. What is the area of the parking lot? Step 1 • X base #s 1.1 x 2.4 = 2.6 Step 2 • add exponents 3 + 3= 1.1 x 3 10 m x 2.4 x 2.6 x 6 10 3 10 2 m 6 m= DID YOU KNOW It’s a metric world The United States is the only western country not presently using the metric system as its primary system of measurement. The only other countries in the world not using metric system as their primary system of measurement are Yemen, Brunei, and a few small islands. DID YOU KNOW In 1906, there was a major effort to convert to the metric system in the United States, but it was opposed by big business and the attempt failed. The Trade Act of 1988 and other legislation declare the metric system the preferred system of weights and measures of the U.S. trade and commerce, call for the federal government to adopt metric specifications, and mandate the Commerce Department to oversee the program. The conversion is currently under way; however, the metric system has not become the system of choice for most Americans’ daily use. Lost in space In September 1999, the United States lost the Mars Climate Orbiter as it approached Mars. The loss of the $125 million spacecraft was due to scientists confusing English units and metric units. DID YOU KNOW Two spacecraft teams, one at NASA’s Jet Propulsion Lab (JPL) in Pasadena, CA, and the other at a Lockheed Martin facility in Colorado, where the spacecraft was built, were unknowingly exchanging some vital information The missing Mars Climate Orbiter in different units. Lost in space DID YOU KNOW The spacecraft team in Colorado used English units of pounds of force to describe small forces needed to adjust the spacecraft’s orbit. The data was shipped via computer, without units, to the JPL, where the navigation team was expecting to receive the data in metric units. The mix-up in units led to the JPL scientists giving the spacecraft’s computer wrong information, which threw the spacecraft off course. This in turn led to the spacecraft entering the Martian Atmosphere, where it The missing Mars Climate Orbiter burned up. DID YOU ALSO KNOW Lost in space On Jan. 3, 1999, NASA launched the $165 million Mars Polar Lander. All radio contact was lost Dec. 3 as the spacecraft approached the red planet. A NASA team that investigated the loss of the Mars Polar Lander concluded a rocket engine shut off prematurely (due to programming error) during landing, leaving the spacecraft to plummet about 130 feet to certain destruction on the Martian surface. The Gimli Glider a mix up in units On July 23, 1983 Air Canada Flight 143 (a brand new Boeing 767) ran out of fuel while en route to Edmonton from Montreal at 26,000 feet. Miraculously the caption was able to land the plane on an abandoned Royal Canadian Air Force Base at Gimli, where the runways were converted into two lane dragstrips for auto racing. No one was killed. The Gimli Glider a mix up in units On July 23, 1983 Air Canada Flight 143 (a brand new Boeing 767) ran out of fuel while en route to Edmonton from Montreal at 26,000 feet. Miraculously the caption was able to land the plane on an abandoned Royal Canadian Air Force Base at Gimli, where the runways were converted into two lane dragstrips for auto racing. No one was killed. SI Units of Measurement (Metric System) p. 16 • LENGTH – straight line btwn 2 pts. –meters (m) • MASS – amt. of matter in object –kilograms (kg) • VOLUME – amt. of space occupied by object –liters (L) Common Metric prefixes & values Prefix Multiple Symbol kilo 1000 k hecto 100 h deca 10 da Base units (meter, liter, gram) 1 m, L, g deci centi 0.1 (1/10) d 0.01 (1/100) c 0.001 (1/1000) milli Why scientists use metrics 2:31 m Kids (kilo, 1,000) Have (hecto, 100) Dazzling (deca, 10) Uniforms (Unit of measurement) During (deci 1/10) Cross Country (centi 1/100) Meets (milli, 1/1,000) 10 mm = _______ cm 100 kg = ________ g 5296 mL = _____________ L Other SI Prefixes (p. 17) Prefix Multiple Symbol Giga Billion (109) G Mega Million (106) M Micro Millionth (10-6) µ Nano Billionth (10-9) n Derived Units p. 16 Quantity Unit Symbol Area Square meter m2 Volume Cubic meter 3 m Density Kilograms per cubic meter kg/m3 VOLUME English vs. Metric Units Which is larger? A. 1 liter or 1 gallon B. 1 liter or 1 quart C. 1 milliliter or 1 fluid ounce 1 gallon = 3.79 liters It would take approximately 3 ¾ 1-liter bottles to equal a gallon. 1 fl oz = 29.573 mL 1- 12oz can of soda = approx 355 mL. 1 quart = 0.946 liters Metric Units Volume is amt of space object occupies Base unit is liter (L). Standard: 1 liter = cubic decimeter Which is larger? A. 1 liter or 1500 milliliters B. 200 milliliters or 1.2 liters Click the image to watch a short video about volume. C. 12 cm3 or 1.2 milliliters Liter Image: http://www.dmturner.org/Teacher/Pictures/liter.gif Measuring Volume Read the measurement based on the bottom of the meniscus or curve. Read at eye-level with level of liquid. What is the volume of liquid in cylinder? __________ What causes the meniscus? A meniscus occurs when molecules of liquid attract molecules of container. Top Image: http://www.tea.state.tx.us/student.assessment/resources/online/2006/grade8/science/images/20graphicaa.gif Bottom Image: http://morrisonlabs.com/meniscus.htm What is the volume of water in each cylinder? Pay attention to the scales for each cylinder. Images created at http://www.standards.dfes.gov.uk/primaryframework/downloads/SWF/measuring_cylinder.swf Measuring Liquid Volume Measuring Solid Volume 8.2 cm 6 cm We can measure the volume of regular object using the formula length x width x height. _____ X _____ X _____ = _____ We can measure the volume of irregular object using water displacement. Amount of H2O with object = ______ About of H2O without object = ______ Difference = Volume = ______ Click here for an online activity about volume. Choose Lessons Volume & Displacement http://resources.edb.gov.hk/~s1sci/R_S1Science/sp/e n/syllabus/unit14/new/testingmain1.htm 9.0 cm MASS Which is larger? English vs. Metric Units 1. 1 Pound or 100 Grams 2. 1 Kilogram or 1 Pound 3. 1 Ounce or 1000 Milligrams 1 pound = 453.6 grams 1 oz gold = 28,349.5 mg 100 kg = 220 pounds Metric Units Mass - amt of matter in object. Base unit is kilogram (kg) Standard: 1 kg = mass of International Prototype Kilogram (IPK), a platinum-iridium cylinder kept by the Bureau International des Poids et Mesures (International Bureau of Weights and Measures [BIPM]) . Which is larger? A. 1 kilogram or 1500 grams C. 12 milligrams or 12 kilograms B. 1200 milligrams or 1 gram D. 4 kilograms or 4500 grams Kilogram Prototype Image - http://en.wikipedia.org/wiki/Kilogram Kilogram Prototype Click the image to watch a short video about mass. Measuring Mass Objects placed on pan Starting with largest weight, move weights on beams until lines match up. Once you have balanced the scale, you add up the amounts on each beam to find the total mass. What would be the mass of the object measured in the picture? _______ + ______ + _______ = ________ g Top Image: http://www.southwestscales.com/Ohaus_Triple_Beam_750-SO.jpg Bottom Image: http://www.regentsprep.org/Regents/biology/units/laboratory/graphics/triplebeambalance.jpg Measuring Mass – Triple-Beam Balance 1st – Place the object to be massed on the pan. 2nd – Slide the large weight to the right until the arm drops below the line. Move the rider back one groove. Make sure it “locks” into place. 3rd – Repeat this process with the top weight. When the arm moves below the line, back it up one groove. 4th – Slide the small weight on the front beam until the lines match up. 5th – Add the amounts on each beam to find the total mass to the nearest tenth (0.1) of a gram. Estimate your final digit to the nearest hundredth (0.01) of a gram Click here to try an online activity. Limits of Measurement Precision gauges how exact a measurement is • How precise are the clocks on the right? • Top analog clock is precise to the minute • Digital clock is precise to the NFL flyovers 4:53 second Accuracy is closeness of a measurement to actual value This clock is running 15 minutes slow…… precise to nearest second BUT time not accurate What are scientific measurements? 9:45 Significant figures (digits) • “Sig Figs” - all digits of known measurement + 1 estimated digit • Answer can only be as precise as least precise measurement in calculation • more precise, more sig figs Sig Fig Rules • ALL non-zero #’s are always significant. • Zeros btwn two sig figs are significant. • Trailing zeros only significant after decimal Not sig figs How many sig figs? 3 sig figs 1) 50.5 4 sig figs 2) 26.25 1 sig fig 3) 500 3 sig figs 4) .050 5) 1.0250 5 sig figs Example • You’re curious about the average time it takes you and your friend to walk to Quik Trip to get a slushie. You take 3 minutes 15 seconds (3.25 min) from your house. Your friend takes 4 minutes 30 seconds (4.5 minutes) from her house. 3.25min + 4.5min = 7.75 total min 7.75min / 2 = 3.875 min This answer has 4 sig figs, but least precise number in calculation has only 2 sig figs (4.5 min) Therefore, answer can only have TWO sig figs Your answer is 3.9 minutes (rounded up) Measuring Temperature • 3 temp scales: – Fahrenheit • H2O freezes @ 32°F • boils @ 212°F – Celsius • H2O freezes @ 0°C • Boils @ 100°C – Kelvin (SI base unit for temp) • 0 K – lowest possible temp (= -273.15°C) • K = °C + 273 1.4 Presenting Scientific Data p. 22 • Scientists organize data using data tables and graphs. Data Tables • Relate the manipulated and responding variables Line Graphs • Show changes in related variables • Manipulated (Independent) variable is plotted on the x-axis. • Responding (Dependent) variable is plotted on the y-axis. Bar Graphs • compares set of measurement s, amounts, or changes Circle Graphs • part to whole • Entire circle is 100% • slices are % that make up 100%