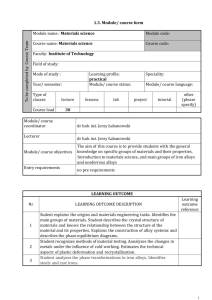
Smart metals: alloys with brains Author Kuang, Justin Author Bio

Smart metals: alloys with brains
Author
Kuang, Justin
Author Bio
Justin Kuang is a sophomore Electrical Engineering student at the University of Southern
California. He enjoys reading science and technology blogs, which is how he stumbled upon the concept of smart metals.
Abstract
Smart metals, or shape memory alloys, are metals that can “remember” certain shapes. These metals have different phases depending on temperature. They are malleable and soft during cooler temperatures, but revert to their remembered shape in warmer temperatures. In addition, they can be “trained” to remember a second shape for their cooler phase. Because of their unique properties, shape memory alloys have found widespread applications and engineers are still working on more.
Keywords
Shape, memory, alloys, smart, metals, Nitinol
Kuang, Justin
WRIT340
Smart metals: alloys with brains
Introduction
Over the years, metals have been widely used for their structural strength, and relatively low weight that comes with it. Their ability to be manipulated into different shapes is also a huge factor to their popularity. A different category of metals, called “smart” metals, takes it a step further with another property: being able to “remember” a shape and revert back to it. We have all bent a paperclip, and then bent it back to its original shape, so what is so special about these metals? Imagine bending the paperclip, and without anybody touching it, seeing the paperclip bend back by itself. These smart metals, also known as shape memory alloys, are capable of exactly this. In a world of science fiction, this could be the discovery that humans have feared: the one that leads to the rise of ruthless self-repairing robots and possibly spell out the end of humanity. However, our world is much more accepting of the technology and has embraced the many applications of its unique properties.
History of smart metals
The first documented research on smart metals, also known as shape memory alloys, took place in 1932. While performing research on an alloy, or combination of different metallic elements, composed of gold and cadmium, scientist Ölander discovered that the alloy possessed seemingly elastic properties. Later in 1938, researchers Greninger and Mooradian working on an alloy of copper and zinc noted that they could alter how bendable the metal was by lower and raising its temperature. Smart metals’ popularity grew in the 1940s to 50s, during which numerous reports described the phenomena of the elastic and thermal properties of the alloys [1]. In 1961, while doing research for the U.S. Navy, researcher Buehler discovered that an alloy of half nickel and half titanium, later called Nitinol, possessed shape memory properties. This discovery happened unintentionally; during a laboratory meeting, a bent piece of the Nitinol alloy was present and was heated with a pipe lighter. To everyone’s surprise, the piece automatically stretched back to its original shape [2]. Widespread application of shape memory alloys began in 1971, when alloys were used to seal the connection between pieces of pipes. Since then, shape memory alloys have been popularized and widely used for their unique properties, and new applications are still being discovered.
How they work
Shape memory alloys have two important properties that lead to their distinct behavior: pseudoelasticity, and a shape memory effect [3]. Elasticity is the ability of an object to stretch and change shapes under stress. Shape memory alloys are able to elastically change shapes, but they differ from other elastic materials since they do not retain that shape permanently. Because changes made to their shape from stress are reversible, they are not truly elastic but instead defined to be pseudoelastic. These alloys also possess a shape memory property, which means
that the metals remember their previous structural arrangements and can revert back to them given the proper conditions.
These alloys achieve their pseudoelastic behavior by using phase changes. Typically when people think of phase changes, they think of the gas, liquid, or solid phases. However, shape memory alloys shift between different kinds of solid phases. This is possible because although the metals remain solids throughout, the particles within the metal are reordered into different arrangements between the solid phases [4]. The two phases inherent to all smart metals are the soft and bendable martensite phase and the hard and uniform austenite phase.
During the martensite phase, the metals are more malleable, meaning they are easier to deform and shape. To illustrate how this phase works, we will define the internal structure of a metal as being made up of many layers of molecules. The molecular structure during the martensite phase is made up of two types of layers: martensite+ and martensite- [5]. These two types are similar, but have one crucial difference in that their molecules are arranged in opposite directions. When the metals are made of even amounts of martensite+ and martensite- layers, there is an overall balance and the metals are in a phase called twinned martensite (See Figure 1b). During this phase if the metals are stretched or bent, the individual layers will take on one of either martensite+ or martensite- types, depending on the direction and orientation of the applied stress.
This stress during the twinned martensite phase converts the metals to a phase called deformed martensite (See Figure 1c). Thus, due to the metal being able to split between two types of layers, the metals in the martensite phase are able to achieve seemingly elastic properties.
The austenite phase is more stable and rigid than the martensite phase, and possesses very organized and cubic arrangement of particles (See Figure 1a). The metals’ shapes during this phase are their “remembered” shapes. The metals’ affinity toward uniformity in this phase is so strong that when the metals transition to this phase, they are able to undo any deformations they might have acquired during their softer martensite phase. Because the metals are able to reorganize their molecules into a strict cubic crystal structure during the austentite phase, they have the ability to “remember” the shape during this phase, achieving a shape memory effect.
Which form the metals take on is mostly dependent on the temperature. At lower temperatures, the metals take on the martensite phase, and for higher temperatures, the austenite phase. For most alloys, the required difference in temperature is not very high, with some being as low as ten degrees Celsius [6]. With heat, metals that are deformed in the martensite phase will revert to their remembered shape in the austenite phase. Upon cooling, the metals’ physical appearance seems to remain the same. However, their molecular structures rearrange themselves back into the twinned martensite phase, becoming soft and malleable again. This transition between having separate bendable layers in the martensite phase and having a hard cubic crystal structure in the austentite phase is what makes shape memory alloys distinct from regular metals.
(a) (b) (c)
Figure 1: Macroscopic and microscopic views of metal structure. (a) Austenite phase with cubic structure.
(b) Twinned martensite phase before any stress. Composed of half martensite+ and half martensite-. (c) Deformed martensite phase after stress applied in one direction.
Source: http://webdocs.cs.ualberta.ca/~database/MEMS/sma_mems/sma.html
Training the metal
Normally, the metals’ are only able to remember a single shape, the one for its austenite phase.
However, engineers have discovered that it is also possible for the metals to remember a shape during their martensite phase [7]. This effect is called the two-way shape memory effect. The previous two properties, superelasticity and a one-way shape memory effect, are inherent properties of all shape memory alloys. The two-way shape memory effect is an acquired trait which the metals must be “trained” to do. The simplest way to train the metals is to shape them a specific way during their martensite phase. The metals are then heated to their austenite phase, and allowed to cool back to martensite, during which they are bent as precisely as possible to their desired shape again. The process is repeated over again and each time, the engineers found that the metals remembered a little bit of the stress applied during the martensite phase. After enough cooling and heating cycles, the metals effectively “remember” their intended shape and will mimic the stressed shape upon cooling without any actual stress applied [3]. At this point, the metals have a shape for both high and low temperatures, and have successfully achieved a two-way memory effect. However, this two-way memory effect is not entirely permanent, and engineers must take caution when attempting to train a metal, as the process is delicate. For example, engineers have discovered that during the heating phase of the training process, if the temperature is too high, the metal may actually forget its shape during the martensite phase, an effect known as “amnesia”.
Applications
Shape memory alloys have found many applications since their discovery in the 1930s, ranging from bathroom uses, to piping, to the production of naval ships. One of the first commercial applications of the alloys was within the U.S. Navy. To connect multiple pieces of pipe together on a ship, a piece of alloy smaller than needed is cooled, usually with liquid nitrogen. The piece is stretched around the pipe ends and then reheated such that it returns to its previous shape, thus tightening up and sealing the connection [8]. Many bathtubs and showers use the same expanding and contracting properties of smart metals to help regulate water temperature. If the stream of warm water is too hot, the alloy will react by automatically blocking off part of the hot water intake to reduce overall water temperature [4].
The memory property of smart metals makes them robust by nature, and thus perfect for situations in which durable and resilient materials are needed. One example is using smart metals for helicopter blades, which are constantly under high stress due to rapid rotations and vibrations.
In optometry, many commercially marketed glasses now use frames that are made out of these alloys. These frames are able to take a high amount of stress and possible deformation and yet revert back to their intended shape to fit tightly around the lenses [4]. In orthodontics, these alloys are being used for dental braces. Bending a smart metal wire and attaching it to teeth will cause the wire to exert constant force on the teeth, thus closing a gap or straightening the teeth.
They are also perfect for dealing with the material stress caused by constant mouth motions [9].
Smart metals have also found their way into lifesaving applications. In fire security systems, smart metal alloys can detect high temperatures in an emergency, react by changing shape or size, and automatically set off an alarm. Or in a factory that deals with flammable or explosive material, they can shut down a volatile process to prevent disasters. In medicine, shape memory alloys are used to make stents, or tubes that are inserted into blood vessels. Using the body’s naturally warm temperature, the metals are able to adapt their size and shape to match the blood vessels. These stents can prevent plaque from completely closing off arteries, thus permitting blood flow and saving people from potential heart attacks or strokes [10]. Shape memory alloys have also found application in large concrete structures such as highways or buildings. Engineers insert wires made of these alloys into concrete slabs (See Figure 2). When the slabs are damaged due to high impact, the wires within reshape themselves, thus partially repairing the concrete
(See Figure 3) [11].
Figure 2: Wires inserted to reinforce a concrete slab. The wires exert forces to close the crack.
Source: http://www.egr.uh.edu/smsl/publication/song_ma_li_%20sma%20civil%20application.pdf
(a) (b)
Figure 3: (a) Reinforced concrete with heavy stress. (b) Concrete after being repaired by shape memory alloy. The crack is significantly smaller.
Source: http://www.egr.uh.edu/smsl/publication/song_ma_li_%20sma%20civil%20application.pdf
The Future
As with most seemingly groundbreaking discoveries, there is a downside to this technology: a steep price tag associated with producing shape memory alloys. The alloys, although versatile and practical, are not as widely used due to their production cost. In order for the alloys to be able to retain their original shapes, they must be cleansed of any impurities in the metals, which greatly increase their production costs. Furthermore, these alloys can be made out of a variety of metals, many of which are not cheap. Currently, the most popular alloy is a combination of half nickel and half titanium called Nitinol, with titanium being expensive to extract. However, research at the University of Zagreb in Croatia has shown that an alloy made out of the cheaper metals copper and aluminum can reduce the production cost substantially while retaining its effectiveness as a shape memory alloy. The group is currently working on methods to mass produce this alloy to make it feasible in commercial settings [12].
What is the future of shape memory alloys? Ideally, the applications will continue to grow and they will be much more widely used. Engineers are still finding new ways to use the unique properties of these alloys: the ability to remember previous shapes, while retaining the strength and other properties of metals. With this much potential, the applications of this technology are endless; they just need to be discovered.
References
[1] Oulu University Library (2000). “Fundamental characterisitics of nickel-titanium shape memory alloy.”
Oulun Yliopisto.
[Online]. Available: http://herkules.oulu.fi/isbn9514252217/html/x317.html
[2] Today’s Machining World (2010, Jan.). “How It Works – Developing a Good Memory:
Nitinol Shape Memory Alloy”. [Online]. Available: http://www.todaysmachiningworld.com/how-it-works-developing-a-good-memorynitinol-shapememory-alloy/
[3] D. Lagoudas. “Detailed Introduction to Shape Memory Alloys.” SmartLab Texas A&M .
[Online]. Available: http://smart.tamu.edu/overview/smaintro/detailed/detailed.html
[4] R. Lin (2008, Feb.). “Shape Memory Alloys and Their Applications.” [Online]. Available: http://www.stanford.edu/~richlin1/sma/sma.html
[5] Mueller, Musolff, Sahota (2005, Apr.). “Shape Memory Alloys”. [Online]. Available: http://www.smaterial.com/SMA/model/model.html
[6] SMA/MEMS Research Group (2001). “Shape Memory Alloys.” [Online]. Available: http://webdocs.cs.ualberta.ca/~database/MEMS/sma_mems/sma.html
[7] S. Swardz. “How Shape Memory Alloys Work.” [Online]. Available: http://depts.washington.edu/matseed/mse_resources/Webpage/Memory%20metals/how_shape_ memory_alloys_work.htm
[8] D. Lagoudas, “Shape Memory Alloys – A Brief History,” in Shape memory alloys: modeling and engineering applications , 1 st
ed.: Springer, 2008, pp. 4-5.
[9] Today’s Machining World (2010, Jan.). “How It Works – Developing a Good Memory:
Nitinol Shape Memory Alloy”. [Online]. Available: http://www.todaysmachiningworld.com/how-it-works-developing-a-good-memorynitinol-shapememory-alloy/
[10] T. Anson (2001, Mar.). “Shape Memory Alloys – Medical Applications.”
Azom . [Online].
Available: http://www.azom.com/article.aspx?ArticleID=134
[11] G. Song (2006, Apr.). “Applications of shape memory alloys in civil structures.” Elsevier .
[Online]. Available: http://www.egr.uh.edu/smsl/publication/song_ma_li_%20sma%20civil%20application.pdf
[12] ScienceDaily (2011, Sep.). “Shape Memory Materials Ready for Mass Production.”
ScienceDaily.
[Online]. Available: http://www.sciencedaily.com/releases/2011/09/110923113445.htm