Assigning Oxidation States
advertisement

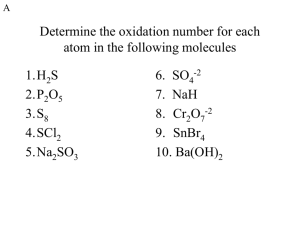
Electrochemistry Ch. 17 Electrochemistry • Generate current from a reaction – Spontaneous reaction – Battery • Use current to induce reaction – Nonspontaneous reaction – Electroplating Oxidation-Reduction Reaction • aka Redox • Transfer of electrons Donor + (reducing agent) (is oxidized) Acceptor (oxidizing agent) (is reduced) Mnemonics are cool! Oxidation Involves Loss of electrons Reduction Involves Gain of electrons Loss of Electrons is Oxidation says Gain of Electrons is Reduction Assigning Oxidation States (1) Covalent bond btw identical atoms => Split electrons evenly (2) Covalent bond btw different atoms => All electrons given to more electronegative atom. (3) For ionic compounds, oxidation states are equal to ionic charge. (4) Oxidation state for an elemental atom is zero. (5) Oxidation state for monatomic ion is the same as the charge. (6) In compounds, fluorine always has an O.S. of -1. (7) Oxygen usually has an O.S. of -2, except when in a peroxide or when in OF2. H2O-2 H2O2-1 +2OF2 (8) With a nonmetal, hydrogen has an O.S. of +1. With a metal, H is assigned an O.S. of -1. NH3+1 LiH-1 (9) The sum of the oxidation states must add up to the overall charge. Examples Assign the oxidation states to each atom of the following compounds. CO2 CH4 K2Cr2O7 Redox Reactions CH4 + O2 CO2 + H2O Which species is oxidized? Which species is reduced? Which species is the oxidizing agent? Which species is the reducing agent? Balancing Redox Reaction • Balance… …# of atoms …# of electrons transferred …overall charge • Types of reactions – Acidic conditions – Basic conditions Redox in Acidic Solutions Cr2O72- + C2H5OH Cr3+ + CO2 1. Assign oxidation states 2. Write half reactions Red: Cr2O72- Cr3+ Ox: C2H5OH CO2 3. Balance elements except H and O Cr2O72- 2Cr3+ C2H5OH 2CO2 4. Balance oxygen by adding H2O Cr2O72- 2Cr3+ + 7H2O 3H2O + C2H5OH 2CO2 5. Balance hydrogen by adding H+ 14H+ + Cr2O72- 2Cr3+ + 7H2O 3H2O + C2H5OH 2CO2 + 12H+ 6. Balance charge by adding electrons 6e- + 14H+ + Cr2O72- 2Cr3+ + 7H2O 3H2O + C2H5OH 2CO2 + 12H+ + 12e7. Equalize the number of electrons 12e- + 28H+ + 2Cr2O72- 4Cr3+ + 14H2O 3H2O + C2H5OH 2CO2 + 12H+ + 12e8. Cancel like terms and add reactions 16H+ + 2Cr2O72- + C2H5OH 4Cr3+ + 2CO2 + 11H2O 9. Check your answer! Balancing in basic solution Following the same algorithm used for acidic solutions through step #8 then… 9. Add the same # of OH- to both sides of equation as there are H+ on one side 10. Combine H+ and OH- on same sides of equation to make H2O 11. Cancel any like terms and check Galvanic Cells • Spontaneous chemistry generating current • Some terms – Reducing agent – Oxidizing agent – Half reactions – Anode – Cathode – Cell potential Building a Galvanic Cell Overall Reaction 8H+(aq) + MnO4-(aq) + 5Fe2+(aq) Mn2+(aq) + 5Fe3+(aq) + 4H2O(l) Half Reactions Reduction: 8H+ + MnO4- + 5e- → Mn2+ + 4H2O Oxidation: 5(Fe2+ → Fe3+ + e-) Reduction: 8H+ + MnO4- + 5e- Mn2+ + 4H2O Oxidation: Fe2+ Fe3+ + e- salt bridge KNO3 Calculating Cell Potential Reduction: 8H+ + MnO4- + 5e- Mn2+ + 4H2O εº(reduction) = 1.51 V Oxidation: 5(Fe2+ Fe3+ + e-) εº(oxidation) = -0.77 V εº(cell) = εº(red) + εº(ox) = 0.74 V Comments on Cell Potential • Potential is an intensive property • DO NOT multiply potential by balancing factor • The º indicates standard conditions – 1.0 M and 1 atm • Potentials references to standard H+ red. 2H+ + 2e- → H2 εº = 0.00 V Fe3+(aq) + Cu(s) → Cu2+(aq) + Fe2+(aq) e- ← Cu e- e- - Fe or Pt +→ salt bridge Cu2+ Oxidation: Cu Cu2+ + 2e- Fe3+ Reduction: Fe3+ + e- Fe2+ Cu2+ + Zn Zn2+ + Cu Demo Line Notation 2Al3+(aq) + 3Mg(s) → 2Al(s) + 3Mg2+(aq) Line Notation II MnO4-(aq) + H+(aq) + ClO3-(aq) → ClO4-(aq) + Mn2+(aq) + H2O(l) Pt(s) │ ClO3-(aq), ClO4-(aq) ║ MnO4-(aq), Mn2+(aq) │ Pt(s) To Review • Full description of galvanic cell requires: – Composition of solutions – Composition of electrodes – Direction of electron flow – Direction of ion flow – Calculation of cell potential – Labels: “anode” and “cathode” Cell Potential and Free Energy w q w q w G q Ch arg e of mole of electrons Faraday F 96485 C F w G q nF mole e G nF G o nF o Reconsidering Cell Potential Given: Al3+ + 3e- Al ΔG1 and ε1 = -1.66V Mg2+ + 2e- Mg ΔG2 and ε1 = -2.37 V Find ε(cell) for: 2Al3+ + 3Mg 2Al + 3Mg2+ G 2G 3G o 3 o 1 o 2 n3 F 2n1 F 3n2 F o 3 o 1 o 2 2n 3n n3 o 3 o 1 1 o 2 2 2(3)(1.66) (3)(2)(2.37) 6 o 3 1.66 2.37 0.71V o 3 Cell Potential and Spontaneity • Can bromine oxidize iodide to iodine? • Can Cr(II) reduce oxygen gas under acidic conditions to produce water? • Can Ag(I) oxidize chloride to chlorine? • Can hydrogen reduce Fe(II) to elemental iron? Non-Standard Cell Potential G G RT ln Q o G nF G nF o o nF nF RT ln Q o RT ln Q nF o 0.0592 o log Q @ 25 C n o Practice Problems Determine ε for the following reaction and conditions: 2Al(s) + 3Mn2+(aq) 2Al3+(aq) + 3Mn (a) [Al3+] = 2.0 M; [Mn2+] = 1.0 M @ 25°C (b) [Al3+] = 1.0 M; [Mn2+] = 3.0 M @ 25°C RT (a) ln Q nF o 0.48V o 2 (8.314)(298) (2.0) 0.48 ln 3 (6)(96485) (1.0) 0.47 V (b) 0.49V Do Worksheet Potential and Equilibrium Cell potential at equilibrium = 0.0 V Q = K at equilibrium RT 0 ln K nF o nF ln K RT o n o log K @ 25 C 0.0592 o Types of Batteries • Lead storage (car battery) • Dry cell battery – Acidic – Alkaline – Rechargeable • Lithium ion battery • Fuel cell Lead Storage Battery Anode: Pb + HSO4- → PbSO4 + H+ + 2eCathode: PbO2 + HSO4- + 3H+ + 2e- → PbSO4 + 2H2O Overall Potential: εº = 2.04 V Dry Cell Battery (acidic) Anode: Zn → Zn2+ + 2eCathode: 2NH4+ + 2MnO2 + 2e- → Mn2O3 + 2NH3 + H2O Overall Potential: εº = 1.5 V Dry Cell Battery (alkaline) Anode: Zn → Zn2+ + 2eCathode: 2MnO2 + H2O + 2e- → Mn2O3 + 2OHOverall Potential: εº = 1.5 V Lithium Ion Battery Anode: Li Li+ + eCathode: MnO2 + Li+ + e- LiMnO2 Cell Potential: εº = 3.6 V Fuel Cell Anode: 2H2(g) + 4OH-(aq) 4H2O + 4eCathode: O2 + 2H2O + 4e- 4OHCell Potential: εº = 1.23 V Corrosion • A significant portion of construction is done to replace corroded materials. Cathode: O2 + 2H2O + 4e- 4OH- ε = 0.40 V Anode: Fe Fe2+ + 2eε = 0.44 V ε(cell) = 0.84 V Corrosion and Acidic Conditions Cathode: O2 + 4H+ + 4e- 2H2O Anode: Fe Fe2+ + 2eε(cell) = 1.67 V ε = 1.23 V ε = 0.44 V Electrolysis • Supply current to perform chemistry • Performed in an electrolytic cell • Stoichiometric relationship btw. charge and chemical amount • Factor-label fun! • Current measured in Ampere = 1 coulomb per second Example Problem I How long will it take to plate out 1.00 kg of aluminum from an aqueous solution of Al3+ using a current of 100.0 A? Al3+ + 3e- Al 1 mol 3 mol e 96485 C 1sec (1000 g Al ) 26.98 g Al 1 mol Al 1 mol e 100 C 107285second = 29.8 h Example Problem II What volume of F2 gas, at 25C and 1.00 atm, is produced when molten KF is electrolyzed by a current of 10.0 A for 2.00 hours? What mass of K metal is produced? At which electrode does each reaction occur? Solution II Molten KF contains K+ and FCathode: K+ + e- K Anode: F- 1/2F2 + e- Volume of F2 60 min 60 s 10.0C 1 mol e 0.5 mol F2 2.00h h min s 96485 C 1 mol e 0.373 mol F2 nRT (0.373)(0.0821)(298) V 9.12 L P 1 Mass of K K+ + e- K F- 1/2F2 + e0.373 mol F2 0.746 mol K (0.746 mol K)(39.10 g/mol) = 29.2 g K Electrolysis in Water Anode: 2H2O O2 + 4H+ + 4eCathode: 4H2O + 4e- 2H2 + 4OH- ε = -1.23V ε = -0.83V 2H2O O2 + 2H2 ε(cell) = -2.06 V Assuming [H+] = [OH-] = 1.0 M Electrolysis in Pure Water • In pure water: [H+] = [OH-] = 1.0 x 10-7 M • Use Nernst equation to determine ε Anode: 107 4 0.0592 0.82V 1.23 log 1 4 Cathode: 107 4 0.0592 0.42V 0.83 log 1 4 Electrolysis in Pure Water • The overall potential for the electrolysis of pure water is -1.24 V. • Need to consider several possible oxidations and reductions when performing electrolysis of aqueous salt solutions. • Consider the electrolysis of 1.0 M NaCl(aq) 1.0 M NaCl(aq) contains: 1.0 M Na+ 1.0 M ClH2O 10-7 M H+ 10-7 M OH- Reducible species: Na+ H + H 2O Oxidizable species: Cl- OH- H 2O Possible Reductions • Using pH = 7.00 Na+ + e- Na H+ + e- 1/2H2 H2O + e- 1/2H2 + OH- ε = -2.71 V ε = -0.414 V** ε = -0.416 V** So, H2 produced at the cathode **Potentials found using Nernst equation Possible Oxidations • Using pH = 7.00 Cl- 1/2Cl2 + eε = -1.36 V 2OH- 1/2O2 + H2O + 2e- ε = -0.814 V** H2O 1/2O2 + 2H+ + 2e- ε = -0.816 V** So, O2 expected to form at the anode. But…