2_05_2003_Wed_f1404_Ch9
advertisement
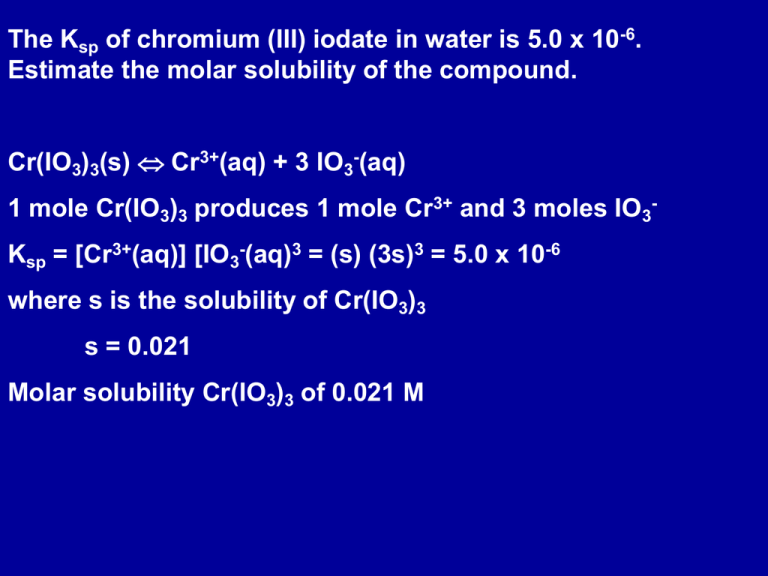
The Ksp of chromium (III) iodate in water is 5.0 x 10-6. Estimate the molar solubility of the compound. Cr(IO3)3(s) Cr3+(aq) + 3 IO3-(aq) 1 mole Cr(IO3)3 produces 1 mole Cr3+ and 3 moles IO3Ksp = [Cr3+(aq)] [IO3-(aq)3 = (s) (3s)3 = 5.0 x 10-6 where s is the solubility of Cr(IO3)3 s = 0.021 Molar solubility Cr(IO3)3 of 0.021 M Precipitation from Solution If equal volumes of aqueous solutions of 0.2 M Pb(NO3)2 and KI are mixed will PbI2(s) precipitate out? Ksp of PbI2 is 1.4 x 10-8 Use the reaction quotient, Q, to predict whether precipitation will occur Pb(NO3)2 (aq) +2 KI(aq) -> PbI2 (s) + 2 KNO3 (aq) Net ionic equation: Pb2+ (aq) + 2I- (aq) -> PbI2 (s) The reverse of this reaction defines Ksp PbI2 (s) Pb2+ (aq) + 2I- (aq) Ksp = [Pb2+ (aq)] [I- (aq)]2 If Q > Ksp precipitation; if Q < Ksp no precipitation Equal volumes of Pb(NO3)2 and KI are mixed On mixing, volume of mixed solution is twice initial volume [Pb2+ (aq)] = 0.2M / 2 = 0.1 M [I- (aq)] = 0.1 M Q = [Pb2+(aq)] [I- (aq)]2 = (0.1)(0.1)2 = 0.001 M Q > Ksp; PbI2(s) precipitates Common Ion Effect Adding NaCl to a saturated solution of AgCl lowers the solubility of AgCl, reducing the amount of Ag+(aq) and Cl- (aq) AgCl(s) Ag+(aq) + Cl-(aq) The common ion effect is the reduction in the solubility of a sparingly soluble salt by the addition of a soluble salt that has an ion in common with it. Example of LeChatelier’s principle. AgCl(s) Ag+(aq) + Cl- (aq) Ksp = 1.6 x 10-10 at 25oC [Ag+ (aq)] = [Cl- (aq)] = 1.3 x 10-5 M concentration of dissolved AgCl = 1.3 x 10-5 M Dissolve AgCl in a solution of 0.10 M NaCl . What is the solubility of AgCl in the NaCl solution? [Cl-(aq)] = 0.10 M Since Ksp at 25oC is a constant, [Ag+(aq)] = Ksp / [Cl- (aq)] = 1.6 x 10-9 M Concentration of dissolved AgCl = 1.6 x 10-9 M Selective Precipitation A mixture of cations in solution can be separated by adding anions with which they form salts with different solubilities. A few drops of a solution of Pb(NO3)2(aq) is added to a solution of KI(aq), yellow PbI2(s) is formed Ca(OH)2(s) (Ksp = 5.5 x 10-6 ) and Mg(OH)2(s) (Ksp = 1.1 x 10-11 ). A sample of sea water contains, among other solutes, the following concentrations of soluble cations: 0.050 M Mg2+(aq) and 0.010 M Ca2+(aq). Determine the order in which each ion precipitates as solid NaOH is added, and give the concentration of OH- when precipitation of each begins. Assume no volume change on addition of solid NaOH. M(OH)2(s) M2+(aq) + 2 OH-(aq) (M = Ca or Mg) [OH- (aq)] = (Ksp / [Ca2+(aq)])0.5 = 0.023 M [OH- (aq)] = (Ksp / [Mg2+(aq)])0.5 = 1.5 x 10-5 M Mg(OH)2(s) will precipitate at a [OH- (aq)] ~ 1.5 x 10-5 M Ca(OH)2(s) will precipitate at a [OH- (aq)] ~ 0.023 M Mg(OH)2(s) precipitates first Dissolving Precipitates The solubility of insoluble compounds can often be increased by addition of acids. ZnCO3 (s) Zn2+ (aq) + CO32- (aq) Adding acid like HNO3(aq) CO32- (aq) + 2 HNO3(aq) -> CO2 (g) + H2O(l) + 2 NO3- (aq) Addition of acids reacts with the anions in solution lowering the concentration of the anion. The insoluble compound then dissolves further to increase the concentration of the anion in solution. Another example of LeChatelier’s principle in action. The solubility of a solid can be increased by removing an ion from solution. Acids can be used to dissolve hydroxides, sulfides, sulfites, or carbonate precipitates. Mg(OH)2(s) Mg2+(aq) + 2 OH-(aq) Ksp = 1.1 x 10-11 In acidic pH concentration of OH- is lowered; increases solubility of the metal hydroxide Estimate the solubility of Fe(OH)3 at 25oC in a solution buffered to a pH of 2.9. Ksp (Fe(OH)3) = 1.1 x 10-36 pOH = 11.1 [OH-(aq)] = 7.94 x 10-12 M Fe(OH)3(s) Fe3+(aq) + 3 OH-(aq) Ksp = 1.1 x 10-36 = [Fe3+(aq)] [OH-(aq)]3 [Fe3+(aq)] = Ksp / [OH-(aq)]3 = 1.1 x 10-36 / (7.94 x 10-12 )3 = 2.2 x 10-3 M = molar solubility of Fe(OH)3 In pure water, molar solubility of Fe(OH)3 is ~ 4.5 x 10-10 M Complex Ion Formation The formation of a complex can remove an ion, affecting the solubility equilibrium. Example: reaction between a Lewis acid such as a metal cation and a Lewis base such as NH3. Ag+(aq) + 2 NH3(aq) Ag(NH3)2+(aq) If NH3 is added to a saturated solution of AgCl, the Ag+ complexes with the NH3, removing the Ag+ from solution, increasing the solubility of AgCl If enough NH3 is added, all the AgCl will dissolve. Both dissolution and complex formation are at equilibrium AgCl(s) Ag+(aq) + Cl-(aq) Ksp = [Ag+(aq)] [Cl-(aq)] Ag+(aq) + 2 NH3(aq) Ag(NH3)2+(aq) Formation constant, Kf: equilibrium constant for complex formation Kf = [Ag(NH3)2+(aq) ] / ([Ag+(aq) ] [NH3(aq) ]2) = 1.6 x 107 at 25oC Calculate the molar solubility of AgCl in 0.10 M NH3(aq) given that Ksp = 1.6 x 10-10 for AgCl and Kf = 1.6 x 107 for Ag(NH3)2+. AgCl(s) Ag+(aq) + Cl-(aq) Ag+(aq) + 2 NH3(aq) Ag(NH3)2+(aq) Overall: AgCl(s) + 2 NH3(aq) Ag(NH3)2+(aq) + Cl-(aq) K = Ksp Kf Molar solubility of AgCl = [Cl-(aq) ] Also, [Ag(NH3)2+(aq)] = [Cl-(aq)] NH3(aq) Ag(NH3)2+(aq) Cl-(aq) Initial 0.10 0 0 Change -2x x x Equilibrium 0.10 - 2x x x K = [Ag(NH3)2+(aq)] [Cl-(aq)] / [NH3(aq)]2 = Ksp Kf = 2.6 x 10-3 x = 4.6 x 10-3 Molar solubility of AgCl is 4.6 x 10-3 M Compare with 1.3 x 10-5 M in pure water Qualitative Analysis Qualitative Analysis involves the separation and identification of ions by techniques such as complex formation, selective precipitation, and control of the pH of a solution. A solution of Pb2+(aq), Hg22+(aq), Ag+ (aq), Cu2+(aq), Zn2+(aq) (1) (2) (3) (1) Add HCl. Precipitate Hg2Cl2, AgCl, PbCl2 (2) Add H2S. Precipitate CuS (3) Make solution basic (add NH3), precipitates ZnS 1) Precipitate of Hg2Cl2, AgCl, PbCl2 Rinse the precipitate in hot water; PbCl2 dissolves Add CrO42- to precipitate Pb2+ as PbCrO4(s) To the Hg2Cl2, AgCl precipitates add NH3 to form Ag(NH3)2+ complex which dissolves. Ag(NH3)2+(aq) + Cl-(aq) + 2 H3O+(aq) AgCl(s) + 2 NH4+(aq) + 2 H2O(l)







