Acid/Base Chemistry
advertisement

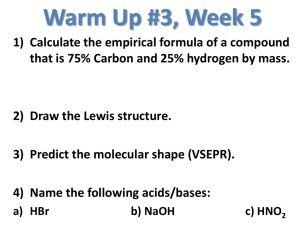
Today… Before Class: Grab a Lab Packet, Safety Goggles, and Apron Our Plan: Inquiry Lab Wrap Up – Venn Diagram Homework (Write in Planner): Inquiry Lab Due next class Mole Day Winners 1st Block – Kate, Tanner, Alondra 2nd Block – Sarah, Jonathan, Lauren, Allyne 5th Block – Annie, Katie, Ashley 6th Block – Emily, Jennifer, Mayra Today… Turn in: Acid/Base Lab if haven’t yet Grab YOUR LAST Calendar, Booklet, & WS Packet You need a TEXTBOOK today! Our Plan: New Calendar Notes Stop the Process/Activity Notes – pH Worksheet #1 Homework (Write in Planner): Worksheet #1 Due next class Chemistry Humor…. Properties of Acids & Bases ACIDS Produce H3O+1 ions when dissolved in water BASES Produce OH-1 ions when dissolved in water Properties of Acids & Bases ACIDS Taste tart or sour BASES Taste Bitter Properties of Acids & Bases ACIDS Corrosive to body tissue BASES Feel Slippery Properties of Acids & Bases ACIDS Turn blue litmus red BASES Turn red litmus blue Properties of Acids & Bases ACIDS Metals react with acids to form hydrogen gas BASES Properties of Acids & Bases ACIDS BASES Properties of Acids & Bases ACIDS BASES Properties of Acids & Bases ACIDS BASES Nomenclature of Acids HCl HF HBr HI Hydrochloric Acid Hydrofluoric Acid Hydrobromic Acid Hydroiodic Acid More Acids HC2H3O2 H2SO4 HNO3 H2CO3 H3PO4 Acetic Acid Sulfuric Acid Nitric Acid Carbonic Acid Phosphoric Acid Nomenclature of Bases Generally, bases are metal hydroxides, so name the metal + hydroxide Examples: – potassium hydroxide NaOH – sodium hydroxide LiOH – lithium hydroxide KOH Real Life Acids Vinegar Aspirin Vitamin C (orange juice) Soft Drinks (Coca Cola) Foods – Citrus Fruits, Tomatoes Real Life Acids Stomach Acid (Bulimia) Fertilizer Car Batteries Acne Face Washes Real Life Bases Antacids Deodorants Plaster Laxatives Baking Soda Real Life Bases Dish Detergent Body Wash (Soap) Lye Oven & Drain Cleaner Bleach STOP Complete the Stop the Process Activity in your notes. Strong Acids/Bases Fight Club Video http://www.youtube.com/watch?v=TfDVL sBXYcM Hydrogen Ions in Water Water molecules, like anything, are in constant, random motion Occasionally the collisions between water molecules are energetic enough to transfer a hydrogen ion from one molecule to another Hydrogen Ions in Water A water molecule that loses a hydrogen ion becomes a negatively charged hydroxide ion (OH-1) A water molecule that gains a hydrogen ion becomes a positively charged hydronium ion (H3O+1) Hydrogen Ions in Water The reaction in which water molecules produce ions is called the self-ionization of water. 2H2O ↔ H3 +1 O + -1 OH The Ionization of Water 2H2O ↔ H3O+1 + OH-1 equilibrium, only 1.0 x 10-7 moles of H3O+1 and OH-1 are formed (at room temperature). So the concentrations of H3O+1 and OH-1 in pure water are 1.0 x 10-7 M. At The Ionization of Water This means the concentrations of H3O+1 and OH-1 are equal in pure water. Any aqueous solution in which [H3O+1] and [OH-1] are equal is called a neutral solution. The Ionization of Water In any aqueous solution, the product of hydronium ions and hydroxide ions equals 1 x 10-14 [H3O+1] [OH-1] = Kw = 1 X 10-14 Brackets indicate concentration Kw represents the ion-product constant of water Acidic & Basic Solutions Not all solutions are neutral If the concentration of hydronium is greater than hydroxide, the solution is ACIDIC If the concentration of hydronium is less than hydroxide, the solution is BASIC (alkaline) pH concentrations of [H3O+1] & [OH-1] can be very small, and expressing concentration in molarity can be a pain, so… Soren Sorensen came up with the pH scale in 1909 The pH scale is from 0-14, 0 being very acidic and 14 being very basic The pH French for pouvoir hydrogene, which means “hydrogen power” Low pH = very acidic High pH = very basic 7 = Neutral Calculating pH To turn such small concentrations into a pH scale, it is necessary to use logarithms Logarithms allow a large range of values to be conveniently expressed as small, nonexponential numbers. Do you remember logarithms from math class? Calculating pH example, log 103 = 3 and log 106 = 6. Although there is a range of three orders of magnitude (10 x 10 x 10 or 1000) between the numbers 103 and 106, the range of the log values is only 3! For Calculating pH (strong acids) To calculate, use: pH = -log[H3O+1] [H3O+1] = hydronium ion concentration WHY? This is because strong acids completely dissociate This means that the molarity of the acid IS THE SAME AS the molarity of +1 H3O Neutral Water For this reason, neutral water has a pH of 7! -7 pH = -log[1 x 10 ] pH = 7 Try typing it in your calculator Try it Out! Complete the pH table in your Note Booklet using your calculator and the formula. The Answers pH 10-pH 0 1 1 0.1 2 0.01 3 0.001 4 0.0001 The Answers pH 10-pH 5 0.00001 6 0.000001 7 0.0000001 8 0.00000001 9 0.000000001 The Answers pH 10-pH 10 1 x 10-10 11 1 x 10-11 12 1 x 10-12 13 1 x 10-13 14 1 x 10-14 pH The numbers in the column 10-pH that you found are the hydronium ion concentration To calculate [H3O+1] = 10-pH pOH pOH is the same as pH, but instead of being a measure of the hydronium ion concentration, it is the measure of the hydroxide ion concentration It is the exact opposite! pOH Low pOH = very basic (alkaline) High pOH = very acidic 7 = neutral Calculating pOH (strong bases) To calculate, use: pOH = -log[OH-1] [OH-1] = hydroxide ion concentration WHY? This is because strong bases completely dissociate This means that the molarity of the base IS THE SAME AS the molarity of OH-1 pOH To -1 [OH ]: calculate -1 -pOH [OH ] = 10 Combining pH and pOH pH +pOH = 14 The Flow Chart What pH is the pH -if log[H O ] the [OH-1]is 3.8 x -4 10 M? 3 +1 pH + pOH = 14 pOH 10-pH [H3O+1] - log[OH-1] 10-pOH [OH-1] Example What is the pOH if the [H3O+1] is - log[H O 6.2 x 10-1 M? 3 pH +1] pH + pOH = 14 pOH 10-pH - log[OH-1] [H3O+1] 10-pOH [OH-1] Example pH - log[H3O+1] What is the [H3O+1] if the [OH-1] is 1.3 x 10-8 M? pH + pOH = 14 pOH 10-pH - log[OH-1] [H3O+1] 10-pOH [OH-1] Try It Out What is the pH if the [OH-1] is - log[H O 3.80 x 10-4 M? 3 pH +1] pH + pOH = 14 pOH 10-pH - log[OH-1] [H3O+1] 10.6 10-pOH [OH-1] Try It Out What is the pOH if the [OH-1] is - log[H O 6.8 x 10-6 M? 3 pH +1] pH + pOH = 14 pOH 10-pH - log[OH-1] [H3O+1] 5.2 10-pOH [OH-1] STOP…Worksheet Time! Complete Worksheet #1 for next class. Wrap Up Properties of Acids & Bases Review Today… Turn in: Get out WS #1 & NOTES & CALCULATOR Our Plan: Review Activities (Properties, Conjugate Acid/Base, pH calculations) Homework #9 – 13/Finish Worksheet Quiz – pH calculations Notes Worksheet #2 Wrap Up – Go Fish Homework (Write in Planner): Worksheet #2 Due next class Are weak acids dangerous? http://www.youtube.com/watch?v=FU0k Vj5X6Dk Review Properties of Acids & Bases Review Conjugate Acid/Base Activity Example H3PO4 Review Answers [H3O+1] 0.079 M 4.0 x 10-8 M 2.0 x 10-5 M 3.5 x 10-3 M pH 1.1 7.4 4.7 2.5 pOH [OH-1] 12.9 6.6 9.3 11.5 1.3 x 10-13 M 2.6 x 10-7 M 5.0 x 10-10 M 3.2 x 10-12 M Homework Help #9 pH - log[H3O+1] pH + pOH = 14 pOH 10-pH - log[OH-1] [H3O+1] 10-pOH [OH-1] Homework Help 10. A solution of pH pH + pOH = 14 pOH strontium hydroxide with a pH of 11.4 is to be prepared. What mass of - log[H3O+1] 10-pH strontium hydroxide would be required to make 1.00 L of - log[OH-1] 10-pOH this solution? [H3O+1] [OH-1] Homework Help 11. Calculate the pH pH + pOH = 14 pOH pH of a solution that contains 5.00 g of HNO3 in 2.00 L of solution. - log[H3O+1] 10-pH - log[OH-1] [H3O+1] 10-pOH [OH-1] Homework Help 12. A hydrochloric pH pH + pOH = 14 pOH acid solution has a pH of 1.70. What is the [H3O+1] in this solution? - log[H3O+1] 10-pH Considering that HCl is a strong acid, what is the HCl - log[OH-1] 10-pOH concentration of the solution. [H3O+1] [OH-1] Homework Help 13. What is the pH pH + pOH = 14 pOH molarity of a solution of the strong base Ca(OH)2 in a - log[H3O+1] 10-pH solution that has a pH of 10.80? - log[OH-1] [H3O+1] 10-pOH [OH-1] Your Turn to Review Complete the table of pH values individually. Check your work with your neighbor. After completing the review, you will take a quiz that looks exactly the same with different numbers! pH of Weak Acids We calculate the pH of weak acids differently because they partially dissociate That means the concentration of the acid IS NOT the same as the [H3O+1] pH of Weak Acids We use something called the Ka (the acid ionization constant) It has been determined by experiments and is different for each acid Determining Ka Ka = [Products] [Reactants] How the pH formula is derived What is the pH of a solution of 0.1 M acetic acid if the Ka for acetic acid is 1.8 x 10-5? CH3COOH + H2O↔ CH3COO-1 + H3O+1 0.1 M n/a X X How the pH formula is derived Ka = [Products] [Reactants] -5 1.8x10 = 2 __x ___ 0.1M x +1 0 ], is the [H3 which is what we need to find pH! How the pH formula is derived CH3COOH + H2O↔ CH3COO-1 + H3O+1 0.1 M n/a X X (0.1M)1.8*10-5= x2 (0.1M) 0.1M √ 1.8*10-6= √x2 1.3 x -3 10 = x= +1 [H3O ] How the pH formula is derived Basically, we multiplied the Ka by the concentration of the acid and took the square root of it. Thus, the equation: Equation for pH weak acids +1 [H3O ] Once = √ Ka [HA] +1 O ], you find [H3 solve for pH just like before! Sample Problem Find the pH of a 0.4 M acetic acid solution. Ka for acetic acid is -5 1.8 x 10 . Sample Problem = √ (0.4M)(1.8 x [H3O+1] = 0.00268 [H3 +1 O ] = -log [H3O+1] pH = -log[0.00268] pH = 2.57 pH -5 10 ) Try It Out! Find the pOH of a 0.33M acetic acid solution. Ka for acetic -5 acid is 1.8 x 10 . Try It Out! = √ (0.33M)(1.8 x [H3O+1] = 0.00243 [H3 +1 O ] = -log [H3O+1] pH = -log[0.00243] pH = 2.61 pH -5 10 ) Not Done Yet! pH + pOH = 14 14 – 2.61 = pOH pOH = 11.39 Let’s do a hard one from the WS #4 What is the concentration of citric acid if the solution has a pH of 2.5? (0.012 M) pH Practice Problems Complete WS # 2 by next class! Wrap Up Go Fish Time! Today… Turn in: WS #2 Our Plan: Worksheet Review Race Quiz Make Sherbet Go Fish Homework (Write in Planner): Nothing Sherbet Activity Make an acid/base reaction in your mouth to create the flavor of sherbet. In a small paper cup combine the following ingredients: 1/2 teaspoon of citric acid crystals 1 teaspoon of icing sugar 1/2 teaspoon of drink crystals 1/4 teaspoon of sodium bicarbonate (baking soda = base) Mix the ingredients with a popsicle stick. Enjoy the sherbet (but not all at once). It foams in your mouth! Wrap Up Go Fish Time! Today… Turn in: Standardize a Base Lab (if you didn’t last class) Our Plan: Demo/Notes Indicator Lab Homework (Write in Planner): Indicator Lab Today Rainbow Indicator Demos http://www.youtube.com/watch?v=VMk3 7PX_bFI http://www.youtube.com/watch?v=PdprN Twb4Ks Indicators Definition: Compounds whose colors are sensitive to pH Their color changes as pH changes Indicators interval – the pH range over which an indicator changes color Examples: phenolphthalein, methyl red, bromthymol blue…. Transition Lab Instructions Step 1 – obtain a test tube rack(s) with 9 test tubes, a tray of indicators, indicators handout, and a lab handout. Lab Instructions Step 2 – Add 10 drops of the chemical to be tested to EACH test tube. Lab Instructions Step 3 – add one drop of each indicator to a separate test tube (do not mix the indicators). Lab Instructions Step 4 – record the color AND pH range indicated by the pH chart in your data table for each indicator. Lab Instructions Step 5 – analyze the data that you collected to write a predicted pH for each substance. Lab Instructions Step 6 – Clean out the test tubes very well in the sink by rinsing multiple times. Repeat the procedure testing each of the items in the table. Lab Instructions 7 – after you have completed the table, answer the questions on the back. I will look at your results and tell you which group came closest to the actual pH values. That lab pair will receive a prize next class! Step The Correct Answers Item Actual pH 7-UP Vinegar Fruit Juice Lime Water Tap Water Soapy Water Ammonia Sour Candy Crushed Aspirin 3.48 2.45 2.64 2.60 6.72 7.17 11.34 2.82 3.24 Today… Get Out: Notes, WS Packet, Calculator Begin Review Problems on p. 19 Our Plan: Demo – Indicator Videos Notes – Neutralization/Titrations Worksheet #3 Standardize a Base Lab Homework (Write in Planner): WS #3 Due Next Class Review – Answer on p. 19 1. 2. 3. 4. What is the [OH-1] of a 0.4 M strong acid solution? 2.5 x 10-14 What is the pOH of a 6 x 10-4 M solution of strong base? 3.22 What is the pH if the pOH is 2.9? 11.1 What is the pOH of a 0.8 M weak acid solution of acetic acid. Ka for acetic acid 1.8 x 10-5 M. 11.57 Acid – Base Demonstration Acid – Base Demonstration Polyprotic Acids Acids such as H2SO4 , H3PO4 or H2CO3 that have more than one hydrogen (proton) to donate. Polyprotic Acids Ionized… 1 mole +1 3 moles H of H3PO4 = -3 1 mole PO4 Neutralization Occurs when equal molar amounts of acid and base are added. In neutralization: Acid + Base --> Salt + Water Neutralization Reaction Example: HCl + NaOH --> NaCl + H2O Neutralization The number of moles of each substance needed depends on the ratio of H+1 to OH-1. If it’s 1:1, the same amount of each is needed. If there are 2 hydrogens to every 1 hydroxide, you would need twice as much base Examples 6.0 _____ moles NaOH with 6.0 moles HCl 2.0 _____ moles H2SO4 with 4.0 moles KOH Examples 9.0 _____ moles H2SO4 with 6.0 moles Al(OH)3 5.0 _____ moles H3PO4 with 15.0 moles LiOH Neutralization Neutralization & Titrations Definitions: Titration: The process in which a standard solution is added to a solution of unknown concentration to determine its concentration. End Point: The point in a titration when the indicator changes color. Titrations What is needed for a titration? 1. A solution whose concentration is known = Standard Solution 2. An accurate way to measure the volume of that solution = Buret 3. A way to determine when the reaction is complete = Indicator Titration What is a titration? Buret filled with standard solution Flask filled with solution of unknown concentration Titration Video Titrations Acid Base Titrations are reactions between strong acids and strong bases. H+1 + OH-1 --> H2O By doing a titration, you can calculate how many moles of acid and base there are. Titrations If +1 H the amount of either or OH-1 are known, the amount of the other can be calculated. (1:1 Ratio) Real Life Applications of Titration 1. 2. 3. 4. 5. 6. 7. 8. Doctors determining ratios for IV drips during hospital stays Machines that monitor blood glucose level Analyzing urine samples Pregnancy tests Pharmacists titrate to match drug prescriptions with a person’s weight, size, condition Food scientists use to determine levels of salt, sugar, vitamins, if cheese and wine are ready for consumption Test water quality and hardness Neutralizing fatty acids in vegetable oil before refining it as biodiesel. Titrations To Calculate: Remember Moles of Molarity? M = moles L Concentration of solution solution Volume of solution Titrations Remember Dilutions? MAVA = MBVB Concentration of Standard Acid Solution Volume of Concentration Volume of Standard of Unknown Unknown Solution Base Solution Solution Example After titrating, you found that 50. mL of 0.10 M HCl was neutralized by 29 mL of NaOH. What was the Molarity of the base? Example MAVA = MBVB 0.10 M 50. mL X Use 29 mL Algebra to solve for x! x = 0.17 M Example 2 After titrating, you found that 48 mL of 0.50 M NaOH was used to neutralize 25 mL of HCl. How many moles of HCl are in the flask, AND what is its concentration? Example MAVA = MBVB x 25 mL 0.50 M48 mL Use Algebra x = 0.96 M to solve for x! Example Continued Now find the number of moles… M = moles L X 0.96 M 0.025 L x = 0.024 moles Try It Out! After titrating, you found that 53 mL of 0.050 M NaOH was used to neutralize 36 mL of HCl. What was the concentration of the HCl? Try It Out! MAVA = MBVB x 36 mL 0.050 M 53 mL Use Algebra to solve for x! x = 0.074 M CONGRATULATIONS! YOU HAVE COMPLETED ALL OF THE CURRICULUM FOR GENERAL CHEMISTRY! Homework Time! Complete WS #3 by next class! Wrap Up What 3 things do you need for a titration? Today… Turn in: Get out WS#3 to check. Our Plan: Math Review Quiz Work Day Missing Assignments/Labs/Quizzes Test Review Slap Jack & Go Fish Review Homework (Write in Planner): Test Review Due Next Class TEST NEXT CLASS! Review Problem Complete the Worksheet #3 Review Problem on p. 19 of your notes. Today… Get Out: Test Review to Check Our Plan: Station Review Test Review Questions Test Begin Antacid Lab Test (UPS Check) Homework (Write in Planner): Nothing Today… Before Class: Goggles/Apron/Clear off Area Our Plan: Antacid Lab Test Homework (Write in Planner): Lab, if not finished Today… Turn in: Textbook Our Plan: Finish Antacid Lab – Record Results on the Board Final Test Review (20 points) Prepare Your Notecard Review Board Game/Flashcards Homework (Write in Planner): Test Review Due Next Class Today… Turn in: Get out Test Review, Notecard Our Plan: Quick Review Take the Final Pencil, Scratch Paper, PT, Notecard, Calculator Relax/Study Homework (Write in Planner): Have a Great Summer! Come back and visit me! I’ll miss you!