Cell Signaling - Lectures For UG-5
advertisement

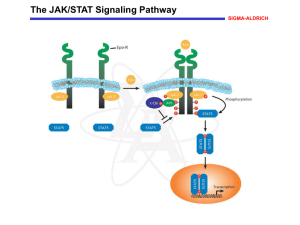
Cell Signaling Lecture 9 JAK/STAT Pathway The JAK-STAT system consists of three main components: (1) a receptor (2) Janus kinase (JAK) (3) Signal Transducer and Activator of Transcription (STAT). transduce a multitude of signals for development and homeostasis in animals, from humans to flies. JAK/STAT pathway JAK activation stimulates: 1. cell proliferation, 2. differentiation 3. cell migration 4. apoptosis. These cellular events are critical to hematopoiesis, immune development, mammary gland development and lactation, adipogenesis, sexually dimorphic growth and other processes. Mutations in JAK/STAT pathway Mutations that constitutively activate or fail to regulate JAK signaling properly cause inflammatory disease, erythrocytosis, gigantism and an array of leukemias. Mutations which slow down or stop JAK/STAT parthway disrupts the process of hematopoiesis, immune development, mammary gland development and lactation, adipogenesis, sexually dimorphic growth and other processes. Cytokines contain 160 amino acids Example 1: Interlukins, essential for proliferation and functioning of T cells and antibody producing B cells of the immune system. T cells: A lymphocyte (WBCs) that matures in the thymus Example 2: Interferon, produced and secreted by certain cell type following virus infection Example 3: Erythropoietin, triggers production of RBCs and by inducing division and differentiation of eryhtroid progenitor cells in the bone marrow Erythropoietin and formation of RBCs Optimal red blood cell (RBC) production requires both erythropoietin (as the controlling factor) and iron (as the raw material). Several factors can impair RBC production; inhibit iron availability, and/or shorten RBC life span . BFU-E, burst-forming unit erythroid; CFU-E, colony-forming unit erythroid. JAK/STAT pathway Intracellular activation of JAK/STAT occurs when ligand binding induces the multimerization of receptor subunits. For some ligands, such as erythropoietin and growth hormone, the receptor subunits are bound as homodimers while, for others, such as interferons and interleukins, the receptor subunits are heteromultimers. For signal propagation through either homodimers or heteromultimers, the cytoplasmic domains of two receptor subunits must be associated with JAK tyrosine kinases. JAKs are distinctive in that they have kinase-homologous domains at the C-terminus. The first is a non-catalytic regulatory domain, whereas the second has tyrosine kinase activity. In mammals, the JAK family comprises four members: JAK1, JAK2, JAK 3 and Tyk2. JAK activation occurs upon ligand-mediated receptor multimerization because two JAKs are brought into close proximity, allowing trans-phosphorylation. The activated JAKs subsequently phosphorylate additional targets, including both the receptors and the major substrates, STATs. STATs are transcription factors that reside in the cytoplasm until activated. The seven mammalian STATs bear a conserved tyrosine residue near the C-terminus that is phosphorylated by JAKs. This phosphotyrosine permits the dimerization of STATs through interaction with a conserved SH2 domain. Phosphorylated STATs enter the nucleus by a mechanism that is dependent on importin α-5 (also called nucleoprotein interactor 1) and the Ran nuclear import pathway. Once in the nucleus, dimerized STATs bind specific regulatory sequences to activate or repress transcription of target genes. Thus the JAK/STAT cascade provides a direct mechanism to translate an extracellular signal into a transcriptional response. JAK/STAT pathway Negative regulators of JAK/STAT pathway there are three major classes of negative regulator: 1.SOCS (suppressors of cytokine signaling) 2.PIAS (protein inhibitors of activated stats) 3.PTPs (protein tyrosine phosphatases) PTP (protein tyrosine phosphatases) The simplest are the protein tyrosine phosphatases, which reverse the activity of the JAKs. The best characterized of these is SHP-1, the product of the mouse motheaten gene. SHP-1 contains two SH2 domains and can bind to either phosphorylated JAKs or phosphorylated receptors to facilitate dephosphorylation of these activated signaling molecules. Other tyrosine phosphatases, such as CD45, appear to have a role in regulating JAK/STAT signaling through a subset of receptors. SOCS (suppressors of cytokine signaling) SOCS proteins are a family of at least eight members containing an SH2 domain and a SOCS box at the C-terminus. In addition, a small kinase inhibitory region located N-terminal to the SH2 domain has been identified for SOCS1 and SOCS3. The SOCS complete a simple negative feedback loop in the JAK/STAT circuitry: activated STATs stimulate transcription of the SOCS genes and the resulting SOCS proteins bind phosphorylated JAKs and their receptors to turn off the pathway. The SOCS can affect their negative regulation by three means. First, by binding phosphotyrosines on the receptors, SOCS physically block the recruitment of signal transducers, such as STATs, to the receptor. Second, SOCS proteins can bind directly to JAKs or to the receptors to specifically inhibit JAK kinase activity. Third, SOCS interact with the elongin BC complex and cullin 2, facilitating the ubiquitination of JAKs and, presumably, the receptors. Ubiquitination of these targets decreases their stability by targeting them for proteasomal degradation. PIAS (protein inhibitors of activated stats) PIAS1, PIAS3, PIASx and PIASy. These proteins have a Zn-binding RING-finger domain in the central portion, a well-conserved SAP (SAF-A/Acinus/PIAS) domain at the Nterminus, and a less-well-conserved carboxyl domain. The latter domains are involved in target protein binding. The PIAS proteins bind to activated STAT dimers and prevent them from binding DNA. The mechanism by which PIAS proteins act remains unclear. Review Paper http://jcs.biologists.org/content/117/8/128 1.full Receptor Tyrosine Kinases Regulate cell proliferation, differentiation, cell survival and collular metabolism The signaling molecules that activate RTK are soluble or membrane bound peptide or protein hormones Examples; Nerve growth factor (NGF) Platelets-derived growth factor (PDGF) Fibroblast growth factor (FGF) Epidermal growth factor (EGF) Like cytokine receptors, RTK signal through a protein tyrosine kinase Unlike cytokine receptors, which associate with a separate cytosolic kinase protein (JAK), RTKs have an intrinsic kinase as part of their cytosolic domain Ligand Binding Leads to Autophosphorylation of RTKs All RTKs comprise an extracellular domain containing a ligand-binding site, a single hydrophobictransmembrane α helix, and a cytosolic domain that includes a region with protein-tyrosine kinase activity. Binding of ligand causes most RTKs to dimerize; the protein kinase of each receptor monomer then phosphorylates a distinct set of tyrosine residues in the cytosolic domain of its dimer partner, a process termed autophosphorylation. Autophosphorylation occurs in two stages. First, tyrosine residues in the phosphorylation lip near the catalytic site are phosphorylated. This leads to a conformational change that facilitates binding of ATP in some receptors (e.g., the insulin receptor) and binding of protein substrates in other receptors (e.g., FGF receptor). The receptor kinase activity then phosphorylates other sites in the cytosolic domain; the resulting phosphotyrosines serve as docking sites for other proteins involved in RTK-mediated signal transduction. Activation of RTK Types of RTK HER1: EGF, HB-EGF, TGFα HER2: does not directly bind a ligand, forms heterodimers with ligand-bound HER1, HER3, HER4 HER3: Neuregulins 1 and 2 (NRG), lacks a functional kinase domain and can signal only when complexed with HER2 HER4: NRG1, NRG2, HB-EGF Down-regulation of RTK Signaling Endocytosis and Lysosomal Degradation Overexpression of HER2 occurs in breats cancer