AP Chemistry - Schedule
Last updated: August 16th, 2011
Unit 1- Welcome Back to Chemistry (Atoms, Molecules, and Ions)
Introductions: Matter and Measurement – Chapter 1
Atoms, Molecules, and Ions – Chapter 2
Unit 2 - Stoichiometry and Predicting Reactions Products
Stoichiometry: Calculations with Chemical Formulas and Equations – Chapter 3
Aqueous Reactions and Solution Stoichiometry – Chapter 4
AP Chem during Academic Lab: October 14th, 2011
Text
Chemistry: The Central Science by Theodore L. Brown, H. Eugene LeMay, Jr., Bruce E.
Bursten, and Catherine J. Murphy, Pearson Prentice Hall, 11th edition, 2009.
The first two units of the course will primarily be review from your previously completed
year long Chemistry course. We will, however, be adding new depth and understanding
to the material. Please note that there is a quiz on Aug 25th, a second quiz on September
8th, and a test on chapters 1-4 on Sept. 16th.
MC Code: MCGAW22955
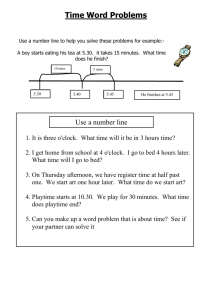
The Class Schedule
Day # Date
1
2
17-Aug
19-Aug
Day
Wed
Fri
3
23-Aug
Tue
4
25-Aug
Thu
5
29-Aug
Mon
Class Work
Getting the class set up
(expectations, processes, HW,
labs, use of academic lab, lab
reports;); Safety
Take Practice Polyatomic Test;
Atomic Structure; Periodic
Table; Molecules & Molecular
compounds; Ions & Ionic
compounds; Set up MC
accounts
Naming Inorganic/Simple
Organic Compounds;
Chemical equations; Patterns
of chemical reactivity;
Quiz (30 min); Review
balancing rxns; formula
weights; Avogadro's number
and the mole
Review Quiz; Empirical
formulas from analysis;
Quantitative information from
balanced equations; Limiting
reactants
Lab
Reading
1.1 - 1.6
Determination of the
Empirical Formula of
Silver Oxide - Day 1
(Vonderbrink #1 - 45
mins)
Determination of the
Empirical Formula of
Silver Oxide - Day 2
(Vonderbrink #1 - 45
mins)
2.1 - 2.7
2.8-2.9; 3.1 - 3.2
3.1-3.4
Finding the Ratio of
Moles of Reactants in
a Chemical Reaction
(Vonderbrink #5 - 50
min)
3.5-3.7
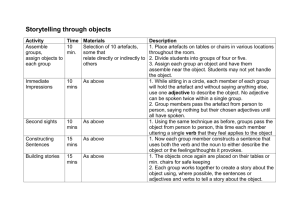
Day # Date
Day
6
31-Aug
Wed
7
6-Sep
Tue
8
8-Sep
Thu
9
12-Sep
Mon
10
11
14-Sep
16-Sep
Wed
Fri
Class Work
Lab
Rxn Practice Test; Properties
of aqueous solutions;
precipitations reactions; acidbase reactions
precipitations reactions; acidbase reactions; OxidationReduction Reactions; Rxn
Practice
Quiz (30 mins) ; Solution
stoichhiometry and chemical
analysis
Liquid
Chromatography
(Vonderbrink #10 - 40
mins)
Reading
4.1 - 4.3
4.4-4.5
4.6
Gravimetric Analysis
of a Metal Carbonate
(Vonderbrink #3 - 90
mins)
Finish up Metal
Carbonate
Review; Practice Test
Test Chapters 1-4 (full block)
The Homework Schedule
MC problems are due the day following the date assigned (e.g., MC2 assigned on the 2nd
day of class is due on the 3rd day of class).
Before-lab questions (BLQ) and procedure (BLP) are due on the day the lab is
performed. After-lab questions (ALQ) are due on the day following the completion of
the lab.
Homework Website: http://masteringchemistry.com
MasteringChemistry Course ID: MCGAW22955
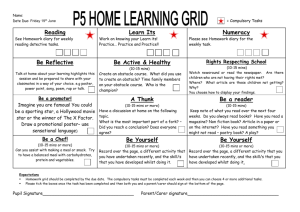
MC Assigned Lab Prep
# Date
1
2
3
17-Aug
19-Aug
23-Aug
Determinatio
n of the
Empirical
Formula of
Silver Oxide Day 1
(Vonderbrink
#1 - 45 mins)
Reading Probs
2.1 - 2.7
1,17, 1.30,
1.59, 1.64,
1.69, 1.74
2.1, 2.2,
2.5, 2.8,
2.11,2.28,
2.31,2.47,
2.58, 2.59
2.8-2.9;
3.1 - 3.2
2.63, 2.64,
2.65, 2.66,
2.67, 2.70,
2.84, 2.101
1.1 - 1.6
ST and Other
ST: Ionic versus Molecular
Compounds; Ions and the
Periodic Table; The Law
of Multiple Proportions
ST: A Formula for
Formulas; Ionic
Compound Nomenclature
and Formulas; Ionic
Compound Formulas
Due
Day
#
Due
Date
2
19-Aug
3
23-Aug
4
25-Aug
MC Assigned Lab Prep
# Date
4
25-Aug
5
29-Aug
6
31-Aug
7
8
Finding the
Ratio of
Moles of
Reactants in
a Chemical
Reaction
(Vonderbrink
#5 - 50 min)
Liquid
Chromatogra
phy
(Vonderbrink
#10 - 40
mins)
6-Sep
8-Sep
9
12-Sep
10
14-Sep
Gravimetric
Analysis of a
Metal
Carbonate
(Vonderbrink
#3 - 90 mins)
Reading Probs
3.1-3.4
3.6, 3.11,
3.14, 3.20,
3.24, 3.25,
3.33, 3.38
4.1 - 4.3
3.8, 3.44,
3.46, 351,
3.57, 3.64,
3.70, 3.72,
3.83, 3.86,
3.93, 3.103,
3.108, 4.1,
4.3, 4.16,
4.18, 4.20,
4.22, 4.29
4.4-4.5
4.30, 4.33,
4.36, 4.42,
4.47, 4.50,
4.52, 4.56
3.5-3.7
4.6
4.43, 4.55
4.62, 4.64,
4.73, 4.81,
4.84, 4.90
3.13, 3.19,
3.52, 3.59,
3.73,
4.93, 4.107,
4.113,
4.100, 4.77,
4.38
ST and Other
ST: Learning
Stoichiometry;
Calculations Using the
Mole
ST: Electrolytes; Acids,
Bases, and Salts
ST: Oxidation States;
Predicting Reaction
Products; Activity Series;
Oxidation-Reduction
Reactions
Due
Day
#
Due
Date
5
29-Aug
6
31-Aug
7
6-Sep
8
8-Sep
ST: Net Ionic Equations;
Reactions in Solution;
Acid-Base Reactions;
Precipitation Reactions
ST: Solution
Stoichiometry;
Precipitation Titration;
Acid-Base Titration
9
12-Sep
10
14-Sep
ST: Electrolytic Properties
and Molarity
11
16-Sep