mercury contamination in s-new england and long island sound, usa
advertisement

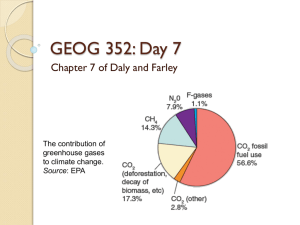
MERCURY CONTAMINATION IN S-NEW ENGLAND AND LONG ISLAND SOUND, USA JOHAN C. VAREKAMP EARTH & ENVIRONMENTAL SCIENCES WESLEYAN UNIVERSITY MIDDLETOWN CT USA Mercury droplets on cinnabar (HgS) MERCURY AND HUMAN HEALTH Mercury has no known biological function and binds tightly to sulfhydryl groups, inhibiting molecular functions -SH •reduces membrane permeability •reacts with and disrupts phosphate bonds in ATP/ADP •replaces cations in important molecules ENVIRONMENTAL CONCERN HUMAN HEALTH CONCERN: HG IS A NEUROTOXIN Victim of the “Minamata Bay” (Japan) tragedy, the first documented disaster of Hg pollution (1954) Exposure to mercury? Eating fish or shellfish Breathing vapors (home, work) Dental work and medical treatments Religious rituals that include Hg inhalation (Santaria in Haiti) Fish Consumption Primary form of human exposure to methylmercury is through fish consumption. Population at greatest risk: small children and pregnant women that consume fish EPA - RfD is 0.1 ug MeHg/day Maximum Hg-in-hair level is 1 ppm Hg EPA limit Wesleyan University study: 35%> EPA limit (nationwide random sample shows 20% above EPA limit) Hg Sources – USA 1998 Oil-Gas Fired power plants Coal-fired power plants Municipal waste incinerators Medical waste incinerators Commercial/Industrial boilers Ore metal smelting Other combustion sources Total combustion related Chlor-alkali production Other sources TOTAL Annual Hg emissions, 106 grams/yr. 0.2 46.9 26.9 14.6 25.8 0.16 10.8 125.4 6.5 12.1 144 Delivery pathways of Hg to the coastal environment • Atmospheric deposition in the watersheds and fluvial transport to the coast • Point source contamination on land with fluvial transport to the coast • Direct discharge through outfall pipes of waste water treatment plants • Dredge and sludge dumping QuickTime™ and a None decompressor are needed to see this picture. Some important forms of Mercury in the environment: 2+ Hg CH3-Hg oxidized organic, charged, lipophilic Hg0 reduced, elemental,volatile CH3-Hg-CH3 organic, volatile, lipophilic aerobic volatilization Hg0 bioaccumulation reduction CH3-Hg+ Hg2+ demethylation (CH3)2-Hg methylation (Sulfate reducing bacteria) anaerobic Hg Transport • Dissolved metals (e.g., in complexes with dissolved organic matter) • Attached to fine particles: • Inorganic • Organic Repositories of metals • Coastal subtidal sediments (delivery mainly by particulate deposition) • Coastal salt marshes and estuarine marshes (delivery mainly by particulate deposition and to some degree through in situ atmospheric deposition) Sediment Cores • Environmental archives that contain contamination records of metals • Record can be blurred by –Chemical mobility in the sediment column –Discontinuous sediment deposition (flood deposits) –Bioturbation Depth, cm 0 10 20 137Cs, dpm/gr 6 5 4 3 1963 2 1 0 30 40 50 Mercury Levels • Normal modern soil background levels for mercury in the northeast USA are around 200 to 300 parts per billion – Mostly due to atmospheric deposition • Sediment samples with higher Hg suggest point sources of Hg in watershed • Hg inventories: total amount of Hg deposited on 1 cm2 over the full pollution period GRAIN SIZE EFFECT ON HG INVENTORIES RG RM SR DB PI FI Mercury profile core Chapman Pond, CT River, CT Mercury profile from core BFB3A, Farm River marsh, Branford, CT 500 GI-1 PTB100 Hg ppb 400 300 200 100 0 1550 1600 1650 1700 1750 1800 Age AD 1850 1900 1950 2000 1750 Knell's Island Core KI 1 Flood Deposits 1500 Hg, ppb 1250 Onset of severe Hg contamination 1000 750 500 250 0 1700 "normal" peak level of Hg contamination in CT onset of 'hatting' industry in Danbury Natural background Hg level 1750 1800 1850 1900 Age, years AD 1950 2000 Core SSaalltt m maarrsshheess BI2 BI3 BI5 PTB/J100 GI E GA GK BFB3a BFA3 BFL8c KI1 F FW W M Maarrsshheess CP3 DM M Mttnn bbooggss TM LL Average Backgr. Onset Peak Peak Modern ppb Hg AD ppb Hg AD ppb Hg 38 58 93 54 35 36 53 55 44 37 54 68 1850 1850 1850 1890 1860 1900 1870 1860 1800 1810 1790 1800 329 243 474 159 398 293 415 420 525 469 333 1544 1970 1939 1974 1956 1960 1959 1972 1967 1940 1944 1997 1964 218 145 206 107 146 97 326 296 236 249 333 177 103 - 1820 - 414 453 1964 358 118 42 44 54 1842 267 356 441 214 276 1962 219 Modern Hg* dep. rate, ng Hg/cm-2 yr-1 13.8 10.8 16.7 % drop Guilford GK GA Peak Hg* d ep. rate, ng Hg cm-2 yr-1 (peak year) 22.8 17.6 (1970) 28.0 (1970) Barn Island BI-2 BI-3 10 8.6 (1965) 12 (1940-1950) 6 6.4 5 40 % 25 % 58 % 35 (1950 -1960) 13 (1930 -1940) Fitzgerald et al. 15 10 57 % 24 % 1-2 Av. : Marsh Core Branford BFA3 BFB3 Modern atmospheric Hg Deposition rate 40 % 40 % 40 % 40 % 15 Mercury Deposition Rates Measured Modern Atmospheric vs. Calculated from Inventories 0 5 10 ng/cm^2 yr Barn Island Pataguanset Guilford Branford Atmosphere THE STILL RIVER, WESTERN CONNECTICUT ~1955 Fowler Island core, Housatonic River Hg ppb Pope Island core, Housatonic River 5000 4500 4000 3500 3000 2500 2000 1500 1000 500 0 0 ~1900 ~ 1800 ~1950 20 40 60 80 100 120 140 Depth cm Floods of 1955 Wooster Square, Danbury The floods of 1955 in Waterbury, CT after two hurricanes hit in a few weeks time Norwalk River Core Hg Concentration vs. Depth of Norwalk Core 6000 Average Hg Concentration (ppb) 1955 5000 4000 3000 1900 2000 1820 1000 0 0 10 20 30 40 50 60 70 80 90 Housatonic River, Still River, Norwalk River: strong evidence for Hg from hatmaking sources Source signals modified by floods The return of the mad hatter Lee Hat Factory Mallory Hat Factory EVERYONE wore hats. Men, women, Danbury, CT "The Hatmaking Capital of the World" Hatmaking started in Danbury ~ 1780 The "carrotage" solution (Hg in nitric acid) is used to make felt from fur The felt-making is done in a steam saturated environment, and the steamcondensate with Hg-nitrate drips from the walls and runs off into the environment Danbury Hat production: 1808 - 100,000 hats / year 1850 - >1 million hats / year were made; 65 hat factories active in Danbury 1920 - >5 million hats / year 1943 - 'carrotage' process outlawed The Carroting Solution …had nothing to do with vegetables. This bright yellow-orange solution of mercury and nitric acid was used to treat animal fur from pelts. It made the fur fibers mat into felt more easily. Men working in Mallory’s carroting room Benedict’s factory initially produced 3 hats per day Background Hg contamination in central and eastern Connecticut, much higher levels of Hg contamination in western Connecticut (Still River and Housatonic River wetlands) How about sediments from Long Island Sound? R/V UCONN Sampling mud HG IN LIS SEDIMENT: GREATEST ENRICHMENTS ON THE WEST SIDE NEAR NEW YORK 700 96017 96024 WLIS 600 Connecticut River Housatonic River Hg ppb 500 400 300 200 100 0 -73.80 -73.55 -73.30 -73.05 -72.80 Longitude -72.55 -72.30 200 Hg 10000 C. perfringens 150 1000 100 100 50 10 0 0 10 20 30 Depth (cm) 40 50 C. perfringens Hg (ppb) core G1C1 1 60 (c) Hg Concentration vs. Depth of WLIS 75GGC1 Core Average Hg Concentration (ppb) 3500 1975 3000 2500 2000 1500 1000 1820 500 0 0 20 40 60 80 100 Average Depth (cm) 120 140 160 180 Core near Execution Rock near NYC - 1975 peak is Hg-rich debris of unknown origin Hg Concentration vs. Depth of B1GGC1 Core 1400 1955 Hg Concentration (ppb) 1200 1000 800 1900 600 400 1820 200 0 0 10 20 30 40 50 60 70 80 90 100 110 120 130 140 150 160 170 180 190 Core in the delta of the Housatonic River Average Depth (cm) 500 A4C1 ~1900 floods? Cu Housatonic River sediment pulse? Pb 400 Zn Metals, ppm/ppb Hg % Clay 300 200 100 0 1800 1850 1900 Age years AD 1950 2000 NYB 600 Hg (ppb) R^2=0.78 Westernmost Sound Central-Western Sound Eastern Sound R^2=0.98 400 200 0 0 1000 2000 3000 4000 5000 6000 C. perfringens (spores/g dry sediment) 7000 First estimate of Hg sources for LIS ~30-35 % from Waste Water Treatment Plants ~20-25% from Housatonic River/Danbury (WLIS) Rest from Connecticut River HG POINT SOURCES AD Housatonic River watershed AD Connecticut River watershed WWTP FINE SEDIMENT TRANSPORT LONG ISLAND SOUND EAST WEST IN SITU AD Natural Background Concentrations Peak Contamination, Atmospheric Deposition Modern Surface Sediments Danbury: Old hatmaking sites/Still River Ponds and wetlands in town Still River Surface samples Core samples near golf course Housatonic River Pope Island Fowlers Island Long Island HR Carting Island Pink House Cove Knells Island / Wheeler marsh Long Island Sound Cores Surface sediment Hg ppb 50 – 100 400 – 700 200 – 300 1000 – 60,000 100 – 1000 1500 – 5500; 70,000 500 – 100,000 1000 – 5000 500 – 2500 100 – 2500 100 – 2200 100 – 1100 100 – 2000 50 – 1200 50 – 700 We have documented extensive Hg contamination in soils and sediments from a known point source: hat-making! How do we get rid of the Hg?? Phytoremediation REMOVAL OF POLLUTANTS THROUGH PLANT UPTAKE: STORAGE IN PLANT FOLLOWED BY PLANT REMOVAL OR FOR HG UPTAKE IN PLANT, REDUCTION TO Hgo AND THEN EMISSION OF VAPOR FROM LEAVES Growth Experiment Brassica rapa P. (Mustard Spinach) 1. Good correlation between Hg in leaves and Hg in spiked soils 2. No correlation between Hg leaves and Hg from ‘field contaminated soils’ 3. Decrease in Hg in leaves over time Hg in Maple Trees Soils with 0.1 -- 75 ppm Hg Hg in leaves increased over time Positive correlation Hg(leaves) with Hg(soil) Mean Hg loss from soils Hg in ‘normal leaves’ minus Hg in ‘MER A leaves’ About 300 microgram Hg / m2 per growing season Ten cm thick soil with 50 ppm Hg-about 103-104 yrs to clean up Conclusions Wetland sediments in CT have peak Hg contamination levels of 400-500 ppb Hg, with values 5090,000 ppb Hg in the Housatonic & Still River Basins Most LIS sediments have 100-650 ppb Hg vs natural concentrations of ~ 50-100 ppb Hg Hg contamination started ~AD 1820-1850, coinciding with the Industrial Revolution (and hatting industry) and raised C.perfringens concentrations anthropogenic signals! Hg contamination has decreased by about 50 % since the 1960-1970's The Danbury hat-making industry has been an important source of Hg for western CT and western LIS, starting ~ 1800 AD The EW increase in Hg concentrations in LIS surface sediments: 1. More “fines” to the West 2. Sediment inputs from the Housatonic River, with ppm levels Hg 3. Hg inputs from WWTP PLANT EXPERIMENTS HG UPTAKE DEPEND ON PLANT SPECIES AND HG SPECIATION IN SOIL HG IN LEAVES FROM MAPLE TREES INCREASES WITH TIME AND REFLECTS SOIL HG PHYTOREMEDIATION WORKS IN PRINCIPLE (MER A PLANTS DO NOT RETAIN MUCH HG IN LEAVES) BUT MAGNITUDE IS SMALL ROLE OF PLANTS IN SOIL HG EMISSION IS NOT YET CLEAR Thanks to CT Sea Grant College Program, CTDEP, USGS and the Mellon Foundation for funding. Much of the field and analytical work was done by Wesleyan University students Beth Goldoff, Kate Lauriat, Bart Kreulen, Billo Jallow and Patrick Welsh. THANKS TO YOU FOR LISTENING