Temperature (°C)
advertisement

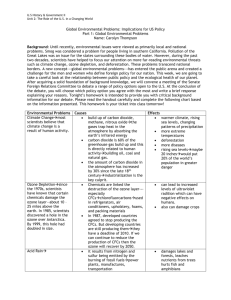
Air Quality and Pollution Control Dr. Martin T. Auer MTU Department of Civil & Environmental Engineering Composition of Clean, Dry (“Pure”) Air Nitrogen Oxygen Argon Carbon dioxide Neon Helium Methane Krypton Hydrogen Nitrous oxide Ozone 78.08 % 20.95 0.93 0.03 18.0 ppm (= 18x10-4 %) 5.2 1.8 1.1 0.5 0.3 0.01 Source: Carnegie Mellon University; Environmental Decision Making, Science & Technology Figure 11.6: Definition of particulate pollutants by size Oxides of Sulfur Source: fossil fuels COOH C CH2 http://www.fieldmuseum.org/research_collections/ecp/ecp_sites/NPI_web/models_coal.htm NH3+ cystine SH Oxides of Sulfur Source: roasting mineral ores International Nickel mines and processes nickel sulfide ores in Sudbury, Ontatio. The Inco Superstack, with a height of 380 m (1,247 ft), is the tallest chimney in the Western hemisphere and the second tallest freestanding chimney in the world after the GRES-2 Power Station in Kazakhstan. heat S O2 SO2 sunlight SO2 O SO3 http://www.flickr.com/photos/23510510@N03/2687642620/ Oxides of Nitrogen Nitrogen dioxide http://www.uncp.edu/home/m cclurem/ptable/co.htm Smog event in Santiago, Chile CO: Concentration / Exposure Figure 11.10: Effect of carbon monoxide on health (After W. Agnew. 1968. Proceedings of the Royal Society A307:153.) Lead Atmospheric lead compared with the exposure standard in Perth, Australia. Lead-based paints were phased out in the 1970s and lead was reduced and then removed from gasoline in the 1980s and 1990s. http://www.soe.wa.gov.au/report/atmosphere/outgoing-issue-lead.html Time Course of Ozone Formation 0.6 hydrocarbons, HC (traffic) Concentration (ppm) 0.5 0.4 0.3 ozone, O3 (photochemical reaction) nitrogen dioxide, NO2 (reaction) 0.2 nitric oxide, NO (traffic) 0.1 0.0 4 6 8 10 12 Time of Day Source: Carnegie Mellon University; Environmental Decision Making, Science & Technology 14 16 18 Lapse Rate, d, (the rate of change of temperature with altitude) Elevation (m) stack d, standard lapse rate -9.8 C/km ambient a, atmospheric lapse rate averaging -6.5 C/km Temperature (°C) http://techalive.mtu.edu/eetext_wg.html UNSTABLE Condition (good mixing) Elevation (m) stack d, standard lapse rate ambient a, atmospheric lapse rate Temperature (°C) STABLE Condition (poor mixing) ambient Elevation (m) stack a, atmospheric lapse rate Temperature (°C) d, standard lapse rate INVERSION (extremely poor mixing) stack Elevation (m) a, atmospheric lapse rate d, standard lapse rate Temperature (°C) ambient Inversion http://www.stuffintheair.com/weather-inversions.html National Ambient Air Quality Standards Criteria Pollutant Standard Type Conc (µg∙m-3) Averaging Period Allowable Exceedance Carbon monoxide Primary and Secondary 10,000 40,000 8 hr 1 hr 1 per year Lead Primary and Secondary 1.5 3 month average Nitrogen Dioxide Primary and Secondary 100 annual mean Ozone Primary and Secondary 210 1 hr 1 per year PM10 Primary and Secondary 150 50 24 hour annual 1 day per year Sulfur Dioxide Primary (Sec) Primary (Sec) 365 (60) 80 (260) 24 hour annual Hydrocarbons Primary and Secondary 160 3 hr maximum National Nonattainment Areas (All Pollutants) National Nonattainment Areas (Ozone) National Nonattainment Areas (Sulfur Diozide) Air Quality Index http://www.deqmiair.org/index.cfm?page=home&sFlush=no Figure 11.6: Definition of particulate pollutants by size Figure 12.2: Cyclone used for dust collection Figure 12.3: Bag filter used for control of particulate air pollutants Figure 12.4: Scrubber Figure 12.5: Electrostatic precipitator used for control of particulate air pollutants Figure 12.9: The effectiveness of various air pollution control devices depends on particle size. Catalytic Converter Noble metals: resistant to corrosion, e.g. platinum and palladium Acid Rain Ozone Hole Global Warming Gaia Hypothesis The Gaia Hypothesis, formulated by James Lovelock in the mid-1960s, proposes that our planet functions as a single organism that maintains conditions necessary for its survival.