2.10 Organic synthesis – Oxidation of alcohols
advertisement

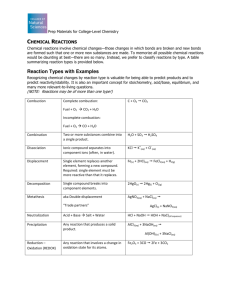
2.10 Organic synthesis – Oxidation of alcohols d. demonstrate an understanding of, and practise, the preparation of an organic liquid (reflux and distillation), eg oxidation of alcohols. Connector: K2Cr2O7 is a mild oxidising agent oxidising agent. a) Balance the ionic half equation below and state whether the Cr2072ion is being oxidised or reduced and justify your answer. a) Describe the colour change for the above reaction. Orange to green b) Draw the skeletal structures of butan-1-ol, butan-2-ol and 2-methylpropan-2-ol, and their reaction products, if any, when they are heated separately with acidified potassium dichromate(VI) solution. c) The oxidation of propan-1-ol to propanal can be written as: CH3CH2CH2OH + [O] CH3CH2CHO + H2O Write the corresponding equation for the complete oxidation of propan-1-ol. How would you carry out these two reactions? Crowe2013 Acidified potassium dichromate(VI) and alcohols K2Cr2O7/H+ 1o OH O O HEAT H K2Cr2O7/H+ H aldehyde K2Cr2O7/H+ 2o HEAT O O ketone OH O H K2Cr2O7/H+ 3o NO REACTION HEAT OH O H HEAT O OH carboxylic acid CH3CH2CH2OH + [O] CH3CH2CHO + H2O Heat propan-1-ol with potassium dichromate (VI) and dilute H2SO4 and immediately distil off the product. CH3CH2CH2OH + 2[O] CH3CH2COOH + H2O Heat propan-1-ol with potassium dichromate (VI) and dilute H2SO4 under reflux. Then collect the propanoic acid by distillation. 1. Draw labelled diagrams of the apparatus used for heating under reflux and distillation. 2. Explain why each process is used, and describe what happens in each process. • The purpose is to thermally accelerate the reaction by conducting it at an elevated temperature (i.e. the solvent's boiling point.). • The reaction will proceed at a constant temperature (i.e. the solvent's boiling point.). •Any vapours given off are cooled back to liquid, and fall back into the reaction vessel • Useful for performing chemical reactions under controlled conditions that require substantial time for completion. heat • The advantage of this technique is that it can be left for a long period of time without the need to add more solvent or fear of the reaction vessel boiling dry as any vapour is immediately condensed in the condenser. • Distillation is a physical separation process. • Distillation is a method of separating mixtures based on differences in their volatilities in a boiling liquid mixture. • See exps 2.13 & 2.14 do reflux and dist parts Preparation of ethanal 3. preventethanal the ethanal 1. To Prevents vapour vapour from escaping by escaping. condensing it. 2. The exothermic reaction would be too vigorous / hazardous / dangerous 3. Why is it necessary to close 1. Explain whyseparating the collection the tap of the funnel container surrounded when all ofisthe sodium by a cooling mixture. dichromate(VI) and ethanol solution has been to the 2. Explain why it isadded important to flask? add the sodium dichromate(VI) and ethanol solution to the acid solution a few drops at a time. 4. Write an equation to show the oxidation of ethanol into ethanal using [O] to represent the oxidizing agent. CH3CH2OH + [O] → CH3CHO + H2O 5. Write an equation to show the oxidation of ethanol into ethanoic acid using [O] to represent the oxidizing agent. CH3CH2OH + 2[O] → CH3COOH + H2O