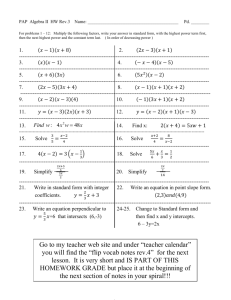
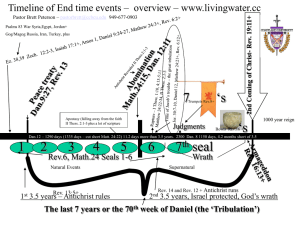
Title page with lifestyle image - Wisconsin Hospital Association
advertisement

Chargemaster Update: Code Changes for 2012 Glenda J. Schuler, RHIT, CPC, CPC-H Updates • Volume of changes potentially impacting the chargemaster Code Changes CPT HCPCS • New Codes • 294 • 348 • Deleted Codes • 101 • • Revised Descriptors • 141 • TOTAL • 536 77 • 425 2 Agenda • • • • • • • • • Category II Vascular Lab Wound Cardiology/Card Cath Lab Pharmacy/IVs Physician and Pro Fees Radiology Laboratory/Blood Bank Anesthesia • • • • • • • • • Infusion Behavioral Health Pulmonary/Respiratory Supplies, Devices & DME Neurology Clinics GI Lab Rehabilitation Services Miscellaneous 3 Potential Changes for 2012 • On Aug. 2, 2011, President Obama signed into law the Budget Control Act of 2011 (BCA). The bill allows the president to increase the debt ceiling by up to $2.8 trillion, but it will reduce the deficit by $2.3 trillion over 10 years, with at least $840 billion coming from discretionary spending cuts over the next decade. • There are two ways the federal deficit can be cut - through "caps" on categories of spending or through a proposed budget hammered out by a "Super Committee" of 12 members of Congress. 4 Status Indicators 5 5 Status Indicators Status Indicator OPPS Payment Status Services furnished to a hospital outpatient that are paid under a fee schedule or payment system other than OPPS, for example: Not paid A under OPPS. Paid by fiscal intermediaries/MACs under a fee schedule or payment system other than OPPS. Services are subject to deductible or coinsurance unless indicated otherwise. ● Ambulance Services ● Clinical Diagnostic Laboratory Services (Not subject to deductible or coinsurance.) ● Non-Implantable Prosthetic and Orthotic Devices ● EPO for ESRD Patients ● Physical, Occupational, and Speech Therapy ● Routine Dialysis Services for ESRD Patients Provided in a Certified Dialysis Unit of a Hospital ● Diagnostic Mammography ● Screening Mammography (Not subject to deductible or coinsurance.) 6 Status Indicators Status Indicator OPPS Payment Status Codes that are not recognized by OPPS when submitted on an B outpatient hospital Part B bill type (12x and 13x). Not paid under OPPS. ● May be paid by fiscal intermediaries/MACs when submitted on a different bill type, for example, 75x (CORF), but not paid under OPPS. C D ● An alternate code that is recognized by OPPS when submitted on an outpatient hospital Part B bill type (12x and 13x) may be available. Inpatient Procedures. Not paid under OPPS. Admit patient. Bill as inpatient. Discontinued Codes. Not paid under OPPS or any other Medicare payment system. 7 Status Indicators Status Indicator OPPS Payment Status Items, Codes, and Services: Not paid by Medicare when submitted on E outpatient claims (any outpatient bill type). ● That are not covered by any Medicare outpatient benefit based on statutory exclusion. ● That are not covered by any Medicare outpatient benefit for reasons other than statutory exclusion. ● That are not recognized by Medicare for outpatient claims but for which an alternate code for the same item or service may be available. ● For which separate payment is not provided on outpatient claims. 8 Status Indicators Status Indicator OPPS Payment Status Corneal Tissue Acquisition; Certain CRNA Services and Hepatitis B F Vaccines. Not paid under OPPS. Paid at reasonable cost. Pass-Through Drugs and Biologicals. Paid under OPPS; separate APC G payment. Pass-Through Device Categories. Separate cost-based pass-through H payment; not subject to copayment. Nonpass-Through Drugs and Nonimplantable Biologicals, Including K Therapeutic Radiopharmaceuticals. Paid under OPPS; separate APC payment. 9 Status Indicators Status Indicator OPPS Payment Status Influenza Vaccine; Pneumococcal Pneumonia Vaccine. Not paid L under OPPS. Paid at reasonable cost; not subject to deductible or coinsurance. Items and Services Not Billable to the Fiscal Intermediary/MAC. Not M paid under OPPS. Items and Services Packaged into APC Rates. Paid under OPPS; N payment is packaged into payment for other services. Therefore, there is no separate APC payment. P Partial Hospitalization. Paid under OPPS; per diem APC payment. 10 Status Indicators Status Indicator OPPS Payment Status STVX-Packaged Codes. Paid under OPPS; Addendum B displays APC Q1 assignments when services are separately payable. (1) Packaged APC payment if billed on the same date of service as a HCPCS code assigned status indicator “S,” “T,” “V,” or “X.” (2) In all other circumstances, payment is made through a separate APC payment. T-Packaged Codes. Paid under OPPS; Addendum B displays APC Q2 assignments when services are separately payable. (1) Packaged APC payment if billed on the same date of service as a HCPCS code assigned status indicator “T.” (2) In all other circumstances, payment is made through a separate APC payment. 11 Status Indicators Status Indicator OPPS Payment Status Codes That May Be Paid Through a Composite APC. Paid under OPPS; Q3 Addendum B displays APC assignments when services are separately payable. Addendum M displays composite APC assignments when codes are paid through a composite APC. (1) Composite APC payment based on OPPS composite-specific payment criteria. Payment is packaged into a single payment for specific combinations of services. (2) In all other circumstances, payment is made through a separate APC payment or packaged into payment for other services. 12 Status Indicators Status Indicator OPPS Payment Status R S T U V X Y Blood and Blood Products. Paid under OPPS; separate APC payment. Significant Procedure, Not Discounted When Multiple. Paid under OPPS; separate APC payment. Significant Procedure, Multiple Reduction Applies. Paid under OPPS; separate APC payment. Brachytherapy Sources. Paid under OPPS; separate APC payment. Clinic or Emergency Department Visit. Paid under OPPS; separate APC payment. Ancillary Services. Paid under OPPS; separate APC payment. Non-Implantable Durable Medical Equipment. Not paid under OPPS. All institutional providers other than home health agencies bill to DMERC. 13 Sources and References CPT and HCPCS code changes Sources for 2012 CPT Codes • 2012 CPT ® Changes, An Insider’s View • Serves as a reference tool to understanding each of the CPT® code changes found in CPT® 2012 codebook. Every new, revised or deleted code, text and guideline change is listed along with a detailed rationale for the change. Immediately know what's new, what's out (deleted) and changed in CPT® for 2012 15 Source for 2012 CPT Coding Changes • Appendix B –Summary of Additions, Deletions and Revisions • Source for all changes in today’s presentation specific for coding changes impacting the facility’s chargemaster 16 OPPS Payments • APC – Ambulatory Payment Classifications • Payments based on CPT codes reported – No CPT code on claim means no additional payment • Payment amounts posted on Addendum B of Medicare’s OPPS website • http://www.cms.gov/HospitalOutpatientPPS/AU/list.asp#TopOfPage 17 Addendum B HCPCS Code Short Descriptor SI APC Relative Weight Payment Rate National Unadjusted Copayment Minimum Unadjusted Copayment 10022 Fna w/image T 0004 4.5843 $315.75 $63.15 19000 Drainage of breast lesion T 0004 4.5843 $315.75 $63.15 19100 Bx breast percut w/o image T 0004 4.5843 $315.75 $63.15 95250 Glucose monitoring cont V 0607 1.8654 $128.48 $25.70 99204 Office/outpatient visit new V 0607 1.8654 $128.48 $25.70 99282 Emergency dept visit V 0613 1.2667 $87.25 G0175 OPPS Service,sched team conf V 0607 1.8654 $128.48 $25.70 G0248 Demonstrate use home inr mon V 0607 1.8654 $128.48 $25.70 G0249 Provide INR test mater/equip V 0607 1.8654 $128.48 $25.70 $20.97 $17.45 18 Source for 2012 HCPCS Coding Changes • CMS Addendum B – HCPCS Long Descriptions, Short Descriptions – http://www.cms.gov/HCPCSReleaseCodeSets/ANHCPC S/list.asp#TopOfPage 19 Appendix G – Moderate Sedation • Summary of codes which Include Moderate (Conscious) Sedation –349 Codes in 2012 –320 Codes in 2011 –301 Codes in 2010 –282 Codes in 2009 20 Chargemaster 2012 and Revenue Codes Hospitals must continue to report HCPCS codes and charges with an appropriate UB revenue code consistent with NUBC requirements. When reporting the appropriate revenue code for services, hospitals should choose the most precise revenue code, or subcode, if appropriate. As NUBC guidelines dictate, “It is recommended that providers use the more detailed subcategory when applicable/available rather than revenue codes that end in “0” (General) or “9” (Other).” Transmittal 1599, Effective October 1, 2008 21 Category II Codes CPT and HCPCS code changes Category II Codes *IMPORTANT INFORMATION* - Updates for CPT® Category II Codes • The American Medical Association, through the CPT Editorial Panel and its Performance Measure Advisory Group, creates CPT Category II codes for use as supplemental tracking codes for quality improvement performance measures. • The AMA publishes the latest Category II codes periodically throughout the year - the latest Category II codes are available at the AMA Web site (www.ama-assn.org/go/cpt) – follow the “Category II Codes” link on the right navigation bar. • Category II codes created after May 2011 are not included in the publications of the CPT® 2012 codebook or the CPT® 2012 data file. They are available at the AMA Web site. 23 Category II Codes • Supplemental tracking codes that can be used for performance measurements – No new instructional notations • Numerous code changes – Additions – Deletions 24 Vascular Lab CPT and HCPCS code changes Vascular Lab SI Rev Code Ind 0920, 092X 0920, 092X X X Action DEL ADD 2011 Code 93875 2012 Code Description Noninvasive physiologic studies of extracranial arteries, complete bilateral study (eg, periorbital flow direction with arterial compression, ocular pneumoplethysmography, Doppler ultrasound spectral analysis) No recommended replacement code Unlisted noninvasive vascular diagnostic 93998 study 26 Wound Clinic CPT and HCPCS code changes Wound Clinic Rev SI Code Ind 0510, 051X N Action ADD 2011 Code 2012 Code Description Near-infrared spectroscopy studies of lower extremity wounds (eg, for 0286T oxyhemoglobin measurement) Near-infrared spectroscopy measures the percentage of hemoglobin oxygen saturation in the microcirculation of tissue up to 3 cm below the skin. The purpose of this study was to describe the measurable response of normal tissue oxygenation in the leg after acute trauma with use of this technique. May be an opportunity for use in Vascular Lab Departments 28 Wound Clinic Rev Code 051X, 076X, 0420, 0430 051X, 076X, 0420, 0430 SI 2011 2012 Ind Action Code Code Description X NEW X NEW Extracorporeal shock wave for integumentary wound healing, high energy, including topical 0299T application and dressing care; initial wound Extracorporeal shock wave for integumentary wound healing, high energy, including topical application and dressing care; each additional wound (List separately in addition to code for 0300T primary procedure) Effective January 1, 2012, not printed in new code book. See also 0019T, 0101T and 0102T 29 Wound Clinic – Skin Products Rev SI Code Ind 2011 Code 2012 Action Code Description Oasis Ultra Tri-Layer Matrix, per square DEL C9365 centimeter Oasis Ultra Tri-Layer Matrix, per square NEW Q4124 centimeter 0636 G 0636 G 0636 0636 0636 0636 0636 0636 G K E E E E NEW NEW NEW NEW NEW NEW 0636 0636 0636 E E N NEW NEW NEW C9366 Q4122 Q4123 Q4125 Q4126 Q4127 Epifix, per square centimeter Dermacell, per square centimenter Alloskin RT, per square centimeter Arthroflex, per square centimeter Memoderm, per square centimeter Talymed, per square centimeter Flex HD or Allopatch HD, per square Q4128 centimeter Q4129 Unite Biomatrix, per square centimeter Q4130 Strattice TM, per square centimeter 30 Cardiology/Cardiac Cath Lab CPT and HCPCS code changes Cardiology Services Rev SI Code Ind Action 0480, 048X S ADD 2011 Code 2012 Code Description Interrogation device evaluation (in person), carotid sinus baroreflex activation system, including telemetric iterative communication with the implantable device to monitor device diagnostics and programmed therapy values, with interpretation and report (eg, battery status, lead impedance, pulse amplitude, pulse width, therapy frequency, pathway mode, burst mode, therapy start/stop 0272T times each day); 32 Carotid Sinus Baroreflex Activation System 33 Cardiology Services Rev SI Code Ind Action 0480, 048X S ADD 2011 Code 2012 Code Description Interrogation device evaluation (in person), carotid sinus baroreflex activation system, including telemetric iterative communication with the implantable device to monitor device diagnostics and programmed therapy values, with interpretation and report (eg, battery status, lead impedance, pulse amplitude, pulse width, therapy frequency, pathway mode, burst mode, therapy start/stop times each day); with programming 0273T 34 Cardiology Services 0480, 048X M ADD 0480, 048X S ADD 0480, 048X S ADD 2012 Code Description External electrocardiographic recording for more than 48 hours up to 21 days by continuous rhythm recording and storage; including recording, scanning analysis with report, review and 0295T interpretation External electrocardiographic recording for more than 48 hours up to 21 days by continuous rhythm recording and storage; recording (includes 0296T connection and initial recording) External electrocardiographic recording for more than 48 hours up to 21 days by continuous rhythm recording and storage; scanning analysis with 0297T report ADD External electrocardiographic recording for more than 48 hours up to 21 days by continuous rhythm 0298T recording and storage; review and interpretation SI Rev Code Ind Action 0480, 048X M 2011 Code 35 Cardiac Catheterization Lab Rev SI Code Ind Action 0480, 048X N DESC 0480, 048X N DESC 2011 2012 Code Code Description Indicator dilution studies such as dye or thermal dilution, including arterial and/or venous catheterization; with cardiac output measurement 93561 (separate procedure) Indicator dilution studies such as dye or thermodilution, including arterial and/or venous catheterization; with cardiac output measurement 93561 (separate procedure) 36 Cardiac Catheterization Lab Rev SI Code Ind Action 0480, 048X N DESC 0480, 048X N DESC 2011 Code 2012 Code Description Indicator dilution studies such as dye or thermal dilution, including arterial and/or venous catheterization; subsequent measurement of 93562 cardiac output Indicator dilution studies such as dye or thermodilution, including arterial and/or venous catheterization; subsequent measurement of 93562 cardiac output 37 Cardiac Catheterization Lab Rev SI Code Ind Action 0480, 048X C ADD 2011 Code 2012 Code Description Percutaneous transcatheter closure of the left atrial appendage with implant, including fluoroscopy, transseptal puncture, catheter placement(s), left atrial angiography, left atrial appendage angiography, 0281T radiological supervision and interpretation Effective January 1, 2012, not printed in book 38 0281T – Left Atrial Appendage • Left atrial appendage is a part of the upper chamber of the heart (left atrium). It has the shape of a pouch and is the place where 90% of clots can be formed in conditions where there is irregular blood flow. Closure of the left atrial appendage could prevent the formation of small clots and protect from stroke 39 0281T – Left Atrial Appendage • • The Watchman (Atritech) is a device that closes off the left atrial appendage (LAA) to minimize the risk of stroke. It is one of several techniques for closing off the left atrial appendage as an alternative to Coumadin or warfarin. Other techniques include removing or clamping off the left atrial appendage during surgery 40 Cardiac Catheterization Lab Rev Code SI Ind Action 0480, 048X N ADD 0480, 048X N ADD 2011 Code 2012 Code Description Intravascular optical coherence tomography (coronary native vessel or graft) during diagnostic evaluation and/or therapeutic intervention, including imaging supervision, interpretation, and report; initial vessel 0291T (List separately in addition to primary procedure) Intravascular optical coherence tomography (coronary native vessel or graft) during diagnostic evaluation and/or therapeutic intervention, including imaging supervision, interpretation, and report; each additional vessel (List separately in addition to primary 0292T procedure) http://www.octnews.org/articles/2050474/lightlab-imaging-c7-xr-fd-octintravascular-imagin/ http://www.abcnews.go.com/GMA/OnCall/video/inside-heart-10698053 41 Hemodynamic monitoring • Continuous monitoring of movement of blood and pressures being exerted in the veins, arteries and chambers of the heart. • Invasive hemodynamic pressure monitoring permits continuous assessment of the status of the critically ill patient and their response to ongoing therapy, providing essential information for more precise diagnosis and prompt correction of a problem. 42 Cardiac Catheterization Lab Rev Code 0480, 048X 0480, 048X SI Ind Action C C 2011 Code ADD 2012 Code Description Insertion of left atrial hemodynamic monitor; complete system, includes implanted communication module and pressure sensor lead in left atrium including transseptal access, radiological supervision and interpretation, and associated injection procedures, 0293T when performed ADD Insertion of left atrial hemodynamic monitor; pressure sensor lead at time of insertion of pacing cardioverterdefibrillator pulse generator including radiological supervision and interpretation and associated injection procedures, when performed (List separately 0294T in addition to code for primary procedure) 43 Cardiac Catheterization Lab Transvenous Procedure Clarification Reference in 2012 code book Provides clarification on appropriate codes to report for pacemaker and implantable cardioverter-defibrillator – Coding example: – Upgrade single chamber system to dual chamber system • Pacemaker – CPT 33233 • ICD - 33241 44 Cardiac Catheterization Lab Rev Code 036X, 048X 036X, 048X 036X, 048X 036X, 048X SI Ind Action T DESC T DESC T DESC T DESC 2011 Code 2012 Code Description Insertion or replacement of permanent pacemaker with 33206 transvenous electrode(s); atrial Insertion of new or replacement of permanent 33206 pacemaker with transvenous electrode(s); atrial Insertion or replacement of permanent pacemaker with 33207 transvenous electrode(s); ventricular Insertion of new or replacement of permanent 33207 pacemaker with transvenous electrode(s); ventricular 45 Cardiac Catheterization Lab Rev Code 036X, 048X 036X, 048X 036X, 048X 036X, 048X SI Ind Action T DESC T DESC T DESC T DESC 2011 Code 2012 Code Description Insertion or replacement of permanent pacemaker with 33208 transvenous electrode(s); atrial and ventricular Insertion of new or replacement of permanent pacemaker with transvenous electrode(s); atrial and 33208 ventricular Insertion or replacement of pacemaker pulse generator only; single chamber, atrial or 33212 ventricular Insertion pacemaker pulse generator only; with 33212 existing single lead 46 Cardiac Catheterization Lab Rev Code 036X, 048X 036X, 048X 036X, 048X SI Ind Action T DESC T DESC T ADD 2011 Code 2012 Code Description Insertion or replacement of pacemaker pulse 33213 generator only; dual chamber Insertion pacemaker pulse generator only; with 33213 existing dual leads Insertion pacemaker pulse generator only; with 33221 existing multiple leads 47 Cardiac Catheterization Lab Rev Code SI Ind Action 036X, 048X T 036X, 048X SI T Rev 036X, 048X 036X, 048X T T 2011 Code 2012 Code Description Repair of single transvenous electrode for a single chamber, permanent pacemaker or single chamber DESC 33218 pacing cardioverter-defibrillator Repair of single transvenous electrode, permanent DESC Action 2011 33218 2012 pacemaker Description or pacing cardioverter-defibrillator Repair of 2 transvenous electrodes for a dual chamber permanent pacemaker or dual chamber DESC 33220 pacing cardioverter-defibrillator Repair of 2 transvenous electrodes for permanent DESC 33220 pacemaker or pacing cardioverter-defibrillator 48 Cardiac Catheterization Lab Rev Code SI Ind Action 036X, 048X T DESC 036X, 048X T DESC 2011 Code 2012 Code Description Insertion of pacing electrode, cardiac venous system, for left ventricular pacing, with attachment to previously placed pacemaker or pacing cardioverterdefibrillator pulse generator (including revision of pocket, removal, insertion, and/or replacement of 33224 generator) Insertion of pacing electrode, cardiac venous system, for left ventricular pacing, with attachment to previously placed pacemaker or pacing cardioverterdefibrillator pulse generator (including revision of pocket, removal, insertion, and/or replacement of 33224 existing generator) 49 Cardiac Catheterization Lab Rev Code 036X, 048X 036X, 048X SI Ind Action T DESC Q3 DESC 2011 Code 2012 Code Description Insertion of pacing electrode, cardiac venous system, for left ventricular pacing, at time of insertion of pacing cardioverter-defibrillator or pacemaker pulse generator (including upgrade to dual chamber system) (List 33225 separately in addition to code for primary procedure) Insertion of pacing electrode, cardiac venous system, for left ventricular pacing, at time of insertion of pacing cardioverter-defibrillator or pacemaker pulse generator (including upgrade to dual chamber system and pocket revision) (List separately in addition to code 33225 for primary procedure) 50 Cardiac Catheterization Lab Rev Code SI Ind Action 036X, 048X T DESC 036X, 048X T DESC 2011 Code 2012 Code Description Repositioning of previously implanted cardiac venous system (left ventricular) electrode (including removal, 33226 insertion and/or replacement of generator) Repositioning of previously implanted cardiac venous system (left ventricular) electrode (including removal, 33226 insertion and/or replacement of existing generator) 51 Cardiac Catheterization Lab Rev SI 2011 2012 Code Ind Action Code Code 036X, 048X T DESC 33233 036X, 048X T DESC 33233 036X, 048X T ADD 036X, 048X T ADD 036X, 048X T ADD Description Removal of permanent pacemaker pulse generator Removal of permanent pacemaker pulse generator only Removal of permanent pacemaker pulse generator with replacement of pacemaker pulse generator; 33227 single lead system Removal of permanent pacemaker pulse generator with replacement of pacemaker pulse generator; dual 33228 lead system Removal of permanent pacemaker pulse generator with replacement of pacemaker pulse generator; 33229 multiple lead system 52 Cardiac Catheterization Lab Rev Code 036X, 048X 036X, 048X Rev 036X, 048X 036X, 048X SI Ind Action 2011 Code 2012 Code Description Insertion of single or dual chamber pacing T DESC 33240 cardioverter-defibrillator pulse generator Insertion of pacing cardioverter-defibrillator pulse T DESC only; with existing single lead SI Action 2011 33240 2012 generator Description Insertion of pacing cardioverter-defibrillator pulse T ADD 33230 generator only; with existing dual lead Insertion of pacing cardioverter-defibrillator pulse T ADD 33231 generator only; with existing multiple lead 53 Cardiac Catheterization Lab Rev Code 036X, 048X 036X, 048X Rev 036X, 048X 036X, 048X 036X, 048X SI Ind Action 2011 Code 2012 Code Description Subcutaneous removal of single or dual chamber T DESC 33241 pacing cardioverter-defibrillator pulse generator Removal of pacing cardioverter-defibrillator pulse T DESC only; SI Action 2011 33241 2012 generator Description Removal of pacing cardioverter-defibrillator pulse generator with replacement of pacing cardioverterT ADD 33262 defibrillator pulse generator; single lead system Removal of pacing cardioverter-defibrillator pulse generator with replacement of pacing cardioverterT ADD 33263 defibrillator pulse generator; dual lead system Removal of pacing cardioverter-defibrillator pulse generator with replacement of pacing cardioverterT ADD 33264 defibrillator pulse generator; multiple lead system 54 Cardiac Catheterization Lab Rev Code 036X, 048X 036X, 048X SI Ind Action T DESC Q3 DESC 2011 Code 2012 Code Description Insertion or repositioning of electrode lead(s) for single or dual chamber pacing cardioverter-defibrillator and 33249 insertion of pulse generator Insertion or replacement of permanent pacing cardioverter-defibrillator system with transvenous 33249 lead(s), single or dual chamber 55 Cardiac Catheterization Lab Rev Code 036X, 048X SI Ind Action B NEW 2011 Code 2012 Code Description Insertion or replacement of a permanent pacing cardioverter-defibrillator system with transvenous lead(s), single or dual chamber with insertion of pacing electrode, cardiac venous system, for left G0448 ventricular pacing 56 Pharmacy and IV CPT and HCPCS code changes Pharmacy and IVs 2011 2012 SI Rev Description Code Ind Action Code Code Anthrax vaccine, for subcutaneous use 0250 E DESC 90581 Anthrax vaccine, for subcutaneous or 90581 intramuscular use 0250 E DESC 58 Pharmacy and IVs Rev Code SI 2011 Ind Action Code 0250 E 0250 E 2012 Code Description Meningococcal conjugate vaccine, serogroups C & Y and Hemophilus influenza b vaccine, tetanus toxoid conjugate (Hib-MenCY-TT), 4dose schedule, when administered to children DESC 90644 2-15 months of age, for intramuscular use Meningococcal conjugate vaccine, serogroups C & Y and Hemophilus influenza b vaccine (Hib-MenCY), 4-dose schedule, when administered to children 2-15 months of age, DESC 90644 for intramuscular use 59 Pharmacy and IVs Rev Code 0636 Rev 0636 SI 2011 Ind Action Code L SI L 2012 Code Description Influenza virus vaccine, pandemic formulation, DEL 90663 H1N1 No replacement provided Action 2011 2012 Description Influenza virus vaccine, split virus, preservativeADD 90654 free, for intradermal use CPT 90663 listed as deleted in Appendix B Transmittal #2174, effective 4/1/11 listed both CPT 90470 and 90663 as deleted, effective 1/1/2011 60 Pharmacy and IVs Rev Code 0636 SI 2011 Ind Action Code G 0636 G Rev SI 0636 G 0636 G DEL C9270 2012 Code Description Injection, immune globulin (Gammaplex), intravenous, Non-lypholized (e.g. Liquid) 500mg Injection, immune globulin (Gammaplex), NEW J1557 intravenous, Non-lypholized (e.g. Liquid) 500mg Action 2011 2012 Description DEL C9272 Injection, denosumab, 1 mg NEW J0897 Injection, denosumab, 1 mg 61 Pharmacy and IVs Rev Code SI 2011 Ind Action Code 0636 G 0636 Rev 0636 0636 G SI G G DEL 2012 Code Description C9274 Crotalidae polyvalent immune fab (Ovine), 1 vial Injection, Crotalidae Polyvalent Immune Fab NEW J0840 (Ovine) Up to 1 gram Action 2011 2012 Description DEL C9276 Injection, cabazitaxel, 1 mg NEW J9043 Injection, cabazitaxel, 1 mg 62 Pharmacy and IVs Rev Code SI 2011 Ind Action Code 0636 G 0636 Rev 0636 0636 2012 Code Description DEL C9277 G SI Injection, alglucosidase alfa (Lumizyme), 1 mg Injection, alglucosidase alfa (Lumizyme), 10 NEW J0221 mg Action 2011 2012 Description G G DEL NEW C9280 Injection, eribulin mesylate, 1 mg J9179 Injection, eribulin mesylate, 0.1 mg 63 Pharmacy and IVs Rev Code 0636 0636 Rev 0636 0636 SI Ind G G SI G G 2011 2012 Action Code Code DEL C9281 Injection, NEW J2507 Injection, Action 2011 2012 DEL C9282 Injection, NEW J0712 Injection, Description pegloticase, 1 mg pegloticase, 1 mg Description ceftaroline fosamil, 10 mg ceftaroline fosamil, 10 mg 64 Pharmacy and IVs Rev Code 0636 0636 Rev 0636 0636 Rev 0250, 0636 0250, 0636 SI Ind G G SI G G SI N 2011 2012 Action Code Code Description DEL C9283 Injection, acetaminophen, 10 mg NEW J0131 Injection, acetaminophen, 10 mg Action 2011 2012 Description DEL C9284 Injection, ipilmumab, 1 mg NEW J9228 Injection, ipilmumab, 1 mg Action 2011 2012 Description Hypertonic saline solution, 50 or 100 mEq, 20 DEL J7130 cc vial N NEW J7131 Hypertonic saline solution, 1 ml 65 Pharmacy and IVs Rev Code 0250 0250, 0636 0250, 0636 Rev 0636 0636 SI 2011 Ind Action Code E G G SI G G 2012 Code Description Injection, von Willebrand factor complex DEL J7184 (human), Wilate, per 100 IU VWF:RCo Injection, von Willebrand factor complex DEL Q2041 (human), Wilate, per 1 IU VWF:RCo Injection, von Willebrand factor complex NEW J7183 (human), Wilate, 1 IU VWF:RCo Action 2011 2012 Description DEL Q2040 Injection, incobotulinumtoxin A, 1 unit NEW J0588 Injection, incobotulinumtoxin A, 1 unit 66 Pharmacy and IVs Rev Code SI 2011 Ind Action Code 0250, 0636 N DEL 0250, 0636 N NEW 2012 Code Description Ondansetron HCl 8 mg, oral, FDA approved prescription antiemetic, for use as a complete therapeutic substitute for an IV antiemetic at the time of chemotherapy treatment, not to Q0179 exceed a 48-hour dosage regimen Ondansetron HCl 1 mg, oral, FDA approved prescription antiemetic, for use as a complete therapeutic substitute for an IV antiemetic at the time of chemotherapy treatment, not to Q0162 exceed a 48-hour dosage regimen 67 Pharmacy and IVs Rev Code SI 2011 Ind Action Code Q2042 2012 Code Description 0636 K DEL Injection, hydroxyprogesterone caproate, 1 mg 0636 Rev 0636 0636 K SI G G NEW J1725 Injection, hydroxyprogesterone caproate, 1 mg Action 2011 2012 Description DEL Q2044 Injection, belimumab, 10 mg NEW J0490 Injection, belimumab, 10 mg 68 Pharmacy and IVs Rev Code SI 2011 Ind Action Code 2012 Code 0636 G NEW 0636 K NEW C9287 Injection, brentuximab vedotin, 1 mg Injection, Alpha 1 Proteinase, Inhibitor J0257 (Human), (Glassia), 10 mg 0250 E NEW 0636 G NEW 0636 K NEW 0250 0636 M K NEW NEW Description J2265 Injection, Minocycline Hydrochloride, 1 mg Injection, Factor XIII (Antihemophilic Factor, J7180 Human), 1 I.U. Hyaluronan or derivative, Gel-One, for intraJ7326 articular injection, per dose Mannitol, administered through an inhaler, 5 J7665 mg J8561 Everolimus, Oral, 0.25 mg 69 Pharmacy and IV – Payment Comparisons HCPCS Code J1835 J1620 J0300 J3030 J1570 J2760 J2725 J9390 J1455 J9070 J0364 J1324 J1644 J1642 Short Descriptor Itraconazole injection Gonadorelin hydroch/ 100 Amobarbital 125 MG inj Sumatriptan succinate / 6 MG Ganciclovir sodium injection Phentolaine mesylate inj Inj protirelin per 250 mcg Vinorelbine tartrate inj Foscarnet sodium injection Cyclophosphamide 100 MG Apomorphine hydrochloride Enfuvirtide injection Inj heparin sodium per 1000u Inj heparin sodium per 10 u SI K K K K K K K K K K K K K K APC 1355 1344 1341 1374 1343 1358 1357 1412 1359 1408 1360 1361 1373 1362 2012 Payment Rate $372.55 $167.25 $113.08 $76.71 $66.58 $53.76 $27.03 $17.45 $14.37 $13.43 $5.12 $0.51 $0.27 $0.18 N N N N N N N N N N N N N N 2011 Payment Rate 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 70 Pharmacy and IV HCPCS Code J2940 J3305 J8650 J9165 Short Descriptor Somatrem injection Inj trimetrexate glucoronate Nabilone oral Diethylstilbestrol injection 2012 Payment SI APC Rate E E E E N N N N 2011 Payment Rate 0.00 0.00 0.00 0.00 Formerly reportable, in 2012 will not be a covered benefit for Medicare 71 Pharmacy and IVs – Payment Updates for 2012 HCPCS Code J1730 J1457 J9212 J0945 J2320 J9265 J2510 C9364 J0735 90476 J0515 C9362 J0630 J0834 90680 J2805 Short Descriptor Diazoxide injection Gallium nitrate injection Interferon alfacon-1 inj Brompheniramine maleate Nandrolone decanoate 50 Paclitaxel injection Penicillin g procaine inj Porcine implant, Permacol Clonidine hydrochloride Adenovirus vaccine type 4 Inj benztropine mesylate Implnt,bon void filler-strip Calcitonin salmon injection Cosyntropin cortrosyn inj Rotovirus vacc 3 dose oral Sincalide injection 2012 Payment SI APC Rate N N N N N N N N N N N N N N N N K K K K K K K G K K K G K K K K 2011 Payment Rate 1.04 2.05 6.88 7.50 9.27 9.43 12.17 17.44 19.32 23.24 25.57 51.87 56.50 68.38 73.07 75.45 72 Physician/Professional Services CPT and HCPCS code changes Physician/Professional Services • Clarification of new versus established patient definitions – Physicians belonging to same group practice: • exact same specialty and subspecialty – Inclusion of Decision Tree in E/M Service Guidelines • Provided as helpful aid in determining whether to report E/M for either new or established patient encounters 74 Physician Services – Inclusion of Time • CPT Codes: – 99218, 99219 and 99220 Code descriptions now inclusive of time physician spends at patient’s bedside and on the patient’s hospital floor or unit 30 minutes, 50 minutes, 70 minutes 75 Physician Services Rev Code 098X 098X SI 2011 Ind Action Code 2012 Code Description Prolonged physician service in the office or other outpatient setting requiring direct (face-to-face) patient contact beyond the usual service; first hour (List separately in addition to code for office or other N DESC 99354 outpatient Evaluation and Management service) Prolonged service in the office or other outpatient setting requiring direct patient contact beyond the usual service; first hour (List separately in addition to code for office or other outpatient Evaluation and N DESC 99354 Management service) New paragraphs added under “Prolonged Services” to clarify use of codes containing revised descriptions 76 Physician Services Rev Code 098X 098X SI 2011 Ind Action Code 2012 Code Description Prolonged physician service in the office or other outpatient setting requiring direct (face-to-face) patient contact beyond the usual service; each additional 30 minutes (List separately in addition to code for N DESC 99355 prolonged physician service) Prolonged service in the office or other outpatient setting requiring direct patient contact beyond the usual service; each additional 30 minutes (List N DESC 99355 separately in addition to code for prolonged service) New paragraphs added under “Prolonged Services” to clarify use of codes containing revised descriptions 77 Physician Services Rev Code 098X 098X SI 2011 Ind Action Code C DESC 99356 C DESC 2012 Code Description Prolonged physician service in the inpatient setting, requiring unit/floor time beyond the usual service; first hour (List separately in addition to code for inpatient Evaluation and Management service) Prolonged service in the inpatient setting or observation, requiring unit/floor time beyond the usual service; first hour (List separately in addition to code for 99356 inpatient Evaluation and Management service) New paragraphs added under “Prolonged Services” to clarify use of codes containing revised descriptions 78 Physician Services Rev Code 098X 098X SI 2011 Ind Action Code 2012 Code Description Prolonged physician service in the inpatient setting, requiring unit/floor time beyond the usual service; each additional 30 minutes (List separately in addition to C DESC 99357 code for prolonged physician service) Prolonged service in the inpatient setting or observation, requiring unit/floor time beyond the usual service; each additional 30 minutes (List separately in C DESC 99357 addition to code for prolonged service) 79 Physician Services Rev Code 096X, 098X 096X, 098X SI 2011 Ind Action Code 2012 Code Description Prolonged evaluation and management service before N DESC 99358 and/or after direct (face-to-face) patient care; first hour Prolonged evaluation and management service before N DESC 99358 and/or after direct patient care; first hour 80 Physician Services Rev Code SI 2011 2012 Ind Action Code Code Description Prolonged evaluation and management service before and/or after direct (face-to-face) patient care; each 096X, additional 30 minutes (List separately in addition to 098X N DESC 99359 code for prolonged physician service) Prolonged evaluation and management service before and/or after direct (face-to-face) patient care; each 096X, additional 30 minutes (List separately in addition to 098X N DESC 99359 code for prolonged service) 81 Physician Services • Prolonged Services With Direct Patient Contact – Direct patient contact is face-to-face and includes additional non-face-to-face services on the patient’s floor or unit in the hospital or nursing facility during the same session • Codes 99354 or 99356 are used to report first hour of prolonged service on a given date, depending on the date of service – Office , outpatient setting, inpatient, observation • Codes 99355 or 99357 are used to report each additional 30 minutes beyond the first hour 82 Physician Services • Prolonged Services Without Direct Patient Contact – Expanded definition for use of codes 99358 and 99359 • Prolonged services not involving direct (face-to-face) care in the office or outpatient setting, nor additional unit/floor time in the hospital or nursing facility setting – During the same session of an evaluation and management service that is beyond the usual physician or other qualified healthcare professional service time – Neonate or newborn critical care • Transfer of services • Referring physician/accepting physician 83 Physician Services • Numerous code revisions for reporting “Physician Quality Reporting System (PQRS)” – Formerly referred to as the Physician Quality Reporting Initiative (PQRI) – Voluntary CMS reporting mechanism used to measure physician quality that will be mandatory as of January 1, 2015. Eligible providers submit quality data to set measures through approved reporting options 84 Radiology Services CPT and HCPCS code changes Radiology Departments Impacted: Angiography/ Interventional & Diagnostic Radiology Radiation Oncology CTA Combination Codes Nuclear Medicine • Diagnostic Renal Angiography • Vena Cava Filter • Paracentesis • Intraoperative radiation treatment delivery procedures • Computerized Tomographic Angiography Abdomen and Pelvis combination CPT • New Hepatobiliary system imaging codes • Pulmonary perfusion/ventilation imaging procedure codes 86 Radiology Bundling • Established by the Affordable Care Act, the Center for Innovation is a new initiative in improving health care for all Americans – Mission is to provide better care at reduced costs through improvement and establish partnerships with providers and professionals • Better health care • Better health • Reduce costs • http://innovations.cms.gov/areas-of-focus/patient-care-models/bundledpayments-for-care-improvement.html 87 Radiology Bundling • CMS initiative to bundle codes and reduce Medicare payments for those procedures performed together greater than 75 percent of the time – Radiology felt first effects of bundling initiatives in 2010 • Myocardial perfusion wall motion • Facet Joint injections • AV shunt dialysis-catheter procedures 88 Radiology Bundling • In 2011, bundling continued with the creation of: – Combined CT abdomen and pelvis codes – Atherectomy codes above the inguinal ligaments including S&I – Lower extremity revascularization procedures • The bundling initiative impacts all areas of medicine, it seems radiology’s codes have collapsed into most surgical procedures 89 Radiology Bundling • In 2012, bundling again has expanded to include: – CTA angiography pelvis and abdomen – Renal Angiography – Inferior vena cava filter – Sacroiliac joint injections – Abdominal paracentesis 90 Renal Angiography Rev Code SI Ind Action 0320 Q2 DEL 0320, 036X Q2 NEW 0320, 036X Q2 NEW 2011 Code 2012 Code Description Angiography, renal, unilateral, selective (including flush aortogram), radiological supervision and 75722 interpretation Selective catheter placement (first-order), main renal artery and any accessory renal artery(s) for renal angiography, including arterial puncture and catheter placement(s), fluoroscopy, contrast injection(s), image postprocessing, permanent recording of images, and radiological supervision and interpretation, including pressure gradient measurements when performed, and flush 36251 aortogram when performed; unilateral Superselective catheter placement (one or more second order or higher renal artery branches) renal artery and any accessory renal artery(s) for renal angiography, including arterial puncture, catheterization, fluoroscopy, contrast injection(s), image postprocessing, permanent recording of images, and radiological supervision and interpretation, including pressure gradient measurements when performed, and flush 36253 aortogram when performed; unilateral 91 Renal Angiography Rev Code SI Ind 2011 Action Code 0320 Q2 DEL 0320, 036X Q2 NEW 0320, 036X Q2 NEW 2012 Code Description Angiography, renal, bilateral, selective (including flush aortogram), radiological supervision and 75724 interpretation Selective catheter placement (first-order), main renal artery and any accessory renal artery(s) for renal angiography, including arterial puncture and catheter placement(s), fluoroscopy, contrast injection(s), image postprocessing, permanent recording of images, and radiological supervision and interpretation, including pressure gradient measurements when performed, and flush 36252 aortogram when performed; bilateral Superselective catheter placement (one or more second order or higher renal artery branches) renal artery and any accessory renal artery(s) for renal angiography, including arterial puncture, catheterization, fluoroscopy, contrast injection(s), image postprocessing, permanent recording of images, and radiological supervision and interpretation, including pressure gradient measurements when performed, and flush 36254 aortogram when performed; bilateral 92 Interventional Radiology Procedures Rev Code SI Ind 0320 N 0320, 036X, 0402 T Rev SI 0320, 036X, 0402 T 2011 Action Code 2012 Code Description Percutaneous placement of IVC filter, radiological DEL 75940 supervision and interpretation Insertion of intravascular vena cava filter, endovascular approach including vascular access, vessel selection, and radiological supervision and interpretation, intraprocedural roadmapping, and imaging guidance (ultrasound and fluoroscopy), NEW 37191 when performed Action 2011 2012 Description Repositioning of intravascular vena cava filter, endovascular approach including vascular access, vessel selection, and radiological supervision and interpretation, intraprocedural roadmapping, and image guidance (ultrasound and fluoroscopy), NEW 37192 when performed 93 Interventional Radiology Procedures Rev Code SI Ind 0320, 036X, 0402 T 2011 Action Code NEW 2012 Code Description Retrieval (removal) of intravascular vena cava filter, endovascular approach including vascular access, vessel selection, and radiological supervision and interpretation, intraprocedural roadmapping, and image guidance (ultrasound and fluoroscopy), 37193 when performed 94 Interventional Radiology Procedures Rev Code 0320, 036X, 0402 0320, 036X, 0402 0320, 036X, 0402 SI Ind 2011 Action Code T DEL T NEW T NEW 2012 Code Description Interruption, partial or complete, of inferior vena cava by suture, ligation, plication, clip, 37620 extravascular, intravascular (umbrella device) Insertion of intravascular vena cava filter, endovascular approach including vascular access, vessel selection, and radiological supervision and interpretation, intraprocedural roadmapping, and imaging guidance (ultrasound and fluoroscopy), 37191 when performed 37619 Ligation of inferior vena cava 95 Interventional Radiology Procedures Rev SI 2011 2012 Code Ind Action Code Code Description 0320, Cons 036X N Sed 36200 Introduction of catheter, aorta Selective catheter placement, arterial system; each 0320, Cons first order abdominal, pelvic, or lower extremity 036X N Sed 36245 artery branch, within a vascular family Selective catheter placement, arterial system; initial 0320, Cons second order abdominal, pelvic, or lower extremity 036X N Sed 36246 artery branch, within a vascular family 96 Interventional Radiology Procedures Rev Code 0320, 036X 0320, 036X SI Ind 2011 Action Code N Cons Sed N Cons Sed 2012 Code Description Selective catheter placement, arterial system; initial third order or more selective abdominal, pelvic, or lower extremity artery branch, within a vascular 36247 family Selective catheter placement, arterial system; additional second order, third order, and beyond, abdominal, pelvic, or lower extremity artery branch, within a vascular family (List in addition to code for 36248 initial second or third order vessel as appropriate) 97 Interventional Radiology Procedures Rev SI Code Ind 0320, 0350, 036X B 0320, 0350, 036X B 2011 Action Code 2012 Code Description Injection procedure for sacroiliac joint, DESC 27096 arthrography and/or anesthetic/steroid Injection procedure for sacroiliac joint, anesthetic/steroid, with image guidance (fluoroscopy or CT) including arthrography DESC 27096 when performed Code 27096 is reported with guided imaging for fluoroscopy or CT. If imaging guidance is not used, see 20552, Injection(s); single or multiple trigger point(s), 1 or 2 muscle(s), reportable with 036X revenue code 98 Interventional Radiology Rev Code SI Ind 0320 Q2 0320, 0350, 036X B 2011 Action Code 2012 Code Description Radiological examination, sacroiliac joint arthrography, radiological supervision and DEL 73542 interpretation Injection procedure for sacroiliac joint, anesthetic/steroid, with image guidance (fluoroscopy or CT) including arthrography DESC 27096 when performed For Medicare, must report G0259 Injection procedure for sacroiliac joint; arthrography G0260 Injection procedure for sacroiliac joint; provision of anesthetic, steroid and/or other therapeutic agent, with or without arthrography 99 Interventional Radiology Rev Code SI Ind 0320 N 0320 N 2011 Action Code 2012 Code Description Fluoroscopic guidance and localization of needle or catheter tip for spine or paraspinous diagnostic or therapeutic injection procedures (epidural, subarachnoid, or sacroiliac joint), including DESC 77003 neurolytic agent destruction Fluoroscopic guidance and localization of needle or catheter tip for spine or paraspinous diagnostic or therapeutic injection procedures (epidural or DESC 77003 subarachnoid) 100 Interventional Radiology Rev Code SI Ind 0320 Q2 0320 Q2 2011 Action Code 2012 Code Description Transluminal balloon angioplasty, peripheral artery other than cervical carotid, renal or other visceral artery, iliac or lower extremity, radiological DESC 75962 supervision and interpretation Transluminal balloon angioplasty, peripheral artery other than renal or other visceral artery, iliac or lower extremity, radiological supervision and DESC 75962 interpretation 101 Interventional Radiology Rev Code SI Ind 0320 N 0320 N 2011 Action Code 2012 Code Description Transluminal balloon angioplasty, each additional peripheral artery other than cervical carotid, renal or other visceral artery, iliac and lower extremity, radiological supervision and interpretation (List separately in addition to code for primary DESC 75964 procedure) Transluminal balloon angioplasty, each additional peripheral artery other than renal or other visceral artery, iliac or lower extremity, radiological supervision and interpretation (List separately in DESC 75964 addition to code for primary procedure) 102 Interventional Radiology Rev Code 0320, 036X 0320, 036X 0320, 036X 0320, 036X 0320, 036X SI Ind T T T T T 2011 Action Code 2012 Code Description Peritoneocentesis, abdominal paracentesis, or DEL 49080 peritoneal lavage (diagnostic or therapeutic); initial Peritoneocentesis, abdominal paracentesis, or peritoneal lavage (diagnostic or therapeutic); DEL 49081 subsequent Abdominal paracentesis (diagnostic or therapeutic; NEW 49082 without imaging guidance Abdominal paracentesis (diagnostic or therapeutic; NEW 49083 with imaging guidance NEW Peritoneal lavage, including imaging guidance, 49084 when performed Remember to “unpanel” or “unexplode” routines which explode the ultrasound, CT guidance or fluoroscopy (e.g. CPT 76942 or 77012) 103 Interventional Radiology Rev Code 036X, 0402, 032X SI Ind T 2011 Action Code 2012 Code Description Cons Sed 47000 Biopsy of liver, needle; percutaneous Continue to report radiology “guidance” code, e.g. ultrasound, Fluoroscopy or CT Code has been added to Appendix G, includes moderate/conscious sedation 104 Interventional Radiology Rev SI 2011 2012 Code Ind Action Code Code Description 0361, 0320, Destruction by neurolytic agent, paravertebral facet 0350 T DEL 64622 joint nerve; lumbar or sacral, single level 0361, 0320, 0350 T NEW Destruction by neurolytic agent, paravertebral facet joint nerve(s), with imaging guidance (fluoroscopy or 64635 CT); lumbar or sacral, single facet joint If fluoroscopic guidance is not utilized, see CPT 64999 105 Interventional Radiology Rev Code SI Ind 2011 Action Code 0361, 0320, 0350 T DEL 0361, 0320, 0350 T NEW 2012 Code Description Destruction by neurolytic agent, paravertebral facet joint nerve; lumbar or sacral, each additional level (List separately in addition to code for primary 64623 procedure) Destruction by neurolytic agent, paravertebral facet joint nerve(s), with imaging guidance (fluoroscopy or CT); lumbar or sacral, each additional facet joint (List separately in addition to code for primary 64636 procedure) If fluoroscopic guidance is not utilized, see CPT 64999 106 Interventional Radiology Rev Code 0361, 0320, 0350 0361, 0320, 0350 SI Ind 2011 Action Code T DEL T NEW 2012 Code Description Destruction by neurolytic agent, paravertebral facet 64626 joint nerve; cervical or thoracic, single level Destruction by neurolytic agent, paravertebral facet joint nerve(s), with imaging guidance (fluoroscopy or 64633 CT); cervical or thoracic, single facet joint If fluoroscopic guidance is not utilized, see CPT 64999 107 Interventional Radiology Rev Code SI Ind 0361, 0320, 0350 T 0361, 0320, 0350 T 2011 Action Code 2012 Code Description Destruction by neurolytic agent, paravertebral facet joint nerve; cervical or thoracic, each additional level (List separately in addition to code for primary DEL 64627 procedure) Destruction by neurolytic agent, paravertebral facet joint nerve(s), with imaging guidance (fluoroscopy or CT); cervical or thoracic, each additional facet joint (List separately in addition to code for primary NEW 64634 procedure) If fluoroscopic guidance is not utilized, see CPT 64999 108 Surgical Procedures - Guidance Rev Code SI Ind 2011 Action Code 032X, 035X, 036X T NEW 032X, 035X, 036X T NEW 2012 Code Description Percutaneous laminotomy/laminectomy (intralaminar approach) for decompression of neural elements, (with or without ligamentous resection, discectomy, facetectomy and/or foraminotomy) any method under indirect image guidance (eg, fluoroscopic, CT), with or without the use of an endoscope, single or multiple levels, unilateral or 0274T bilateral; cervical or thoracic Percutaneous laminotomy/laminectomy (intralaminar approach) for decompression of neural elements, (with or without ligamentous resection, discectomy, facetectomy and/or foraminotomy) any method under indirect image guidance (eg, fluoroscopic, CT), with or without the use of an endoscope, single or multiple levels, unilateral or 0275T bilateral; lumbar 109 Surgical Procedures - Guidance Rev Code SI Ind 0920, 0929 S 0920, 0929 S ADD 2012 Code Description Percutanous or open implantation of neurostimulator electrode array(s), subcutaneous (peripheral subcutaneous field stimulation), including imaging guidance, when performed, cervical, thoracic or lumbar; for trial, including 0282T removal at the conclusion of trial period ADD Percutanous or open implantation of neurostimulator electrode array(s), subcutaneous (peripheral subcutaneous field stimulation), including imaging guidance, when performed, cervical, thoracic or lumbar; permanent, with 0283T implantation of a pulse generator Action 2011 Code 110 Diagnostic Radiology Rev Code 0320 0320 Rev 0320 SI Ind X X SI N 2011 2012 Action Code Code Description DESC 70355 Orthopantogram DESC 70355 Orthopantogram (eg, panoramic x-ray); Action 2011 2012 Description DEL Insertion pacemaker, fluoroscopy and radiography, 71090 radiological supervision and interpretation New parenthetical instructions in 2012 narrative specific for fluoroscopic guidance for lead insertion, replacement or revisions 111 Diagnostic Radiology Rev Code SI Ind 0320 X 0320 Rev X SI 0320 X 0320 X 2011 Action Code 2012 Code Description Radiologic examination, spine, lumbosacral; DESC 72114 complete, including bending views Radiologic examination, spine, lumbosacral; complete, including bending views, minimum of 6 DESC 72114 views Action 2011 2012 Description Radiologic examination, spine, lumbosacral, DESC 72120 bending views only, minimum of 4 views Radiologic examination, spine, lumbosacral, DESC 72120 bending views only, 2 of 3 views 112 Diagnostic Radiology Rev Code SI Ind 035X S 035X X 2011 Action Code DEL DEL 2012 Code Description Computed tomography, bone mineral density study, 1 or more sites; appendicular skeleton (peripheral) 77079 (eg, radius, wrist, heel) See Existing 77078, 77080-77081 77083 Radiographic absorptiometry (eg, photodensitometry, radiogrammetry), 1 or more sites See Existing 77080-77082 113 Radiation Oncology Rev Code SI Ind 2011 Action Code 0333 N NEW 0333 N NEW 2012 Code Description Intraoperative radiation treatment delivery, x-ray, 77424 single treatment session Intraoperative radiation treatment delivery, 77425 electrons, single treatment session 0333 N NEW 77469 Intraoperative radiation treatment management 114 Radiation Oncology Rev Code SI Ind 0333 S 0333 S 2011 Action Code 2012 Code Description Special treatment procedure (eg, total body irradiation, hemibody radiation, per oral, DESC 77470 endocavitary or intraoperative cone irradiation) Special treatment procedure (eg, total body irradiation, hemibody radiation, per oral or DESC 77470 endocavitary irradiation) 115 CT Abdomen & Pelvis Payment Adjustments 2012 HCPCS Code Short Descriptor 74176 Ct abd & pelvis 74177 Ct abd & pelv w/contrast 74178 Ct abd & pelv 1/> regns SI APC Q3 0331 Q3 0334 Q3 0334 Relative Weight 5.7930 8.2987 8.2987 2012 Payment Rate $405.60 $581.04 $581.04 N a 2011 t Payment Payment i Rate Difference Q3 193.85 211.75 Q3 299.81 281.23 Q3 334.24 246.80 116 MRI Contrast Rev Code SI Ind 0254, 0255, 0636 N 2011 Action Code 2012 Code Description NEW A9585 Injection, gadobutrol, 0.1 ml 117 Computerized Tomographic Angiography (CTA) • Continue to report using: – 74175 Computed tomographic angiography, abdomen, with contrast material(s), including noncontrast images, if performed, and image postprocessing – 72191 Computed tomographic angiography, pelvis, with contrast material(s), including noncontrast images, if performed, and image postprocessing • New for 2012: Rev Code 0350, 0352, 0359 SI Ind S 2011 Action Code 2012 Code Description NEW Computed tomographic angiography, abdomen and pelvis, with contrast material(s), including noncontrast images, if performed, and image 74174 postprocessing 118 Nuclear Medicine Rev Code SI Ind 0340, 034X Rev S SI 0340, 034X S 2011 Action Code 2012 Code Description Liver function study with hepatobiliary agents, with DEL 78220 images Action 2011 2012 serial Description Hepatobiliary ductal system imaging, including gallbladder, with or without pharmacologic intervention, with or without quantitative DEL 78223 measurement of gallbladder function 0340, 034X S NEW 0340, 034X S NEW Hepatobiliary system imaging, including gallbladder 78226 when present; Hepatobiliary system imaging, including gallbladder when present; with pharmacologic intervention, including quantitative measurement(s) when 78227 performed 119 Nuclear Medicine Rev Code SI Ind 2011 Action Code 036X, 034X Q1 DESC 38792 036X, 034X Q1 DESC 2012 Code Description Injection procedure; for identification of sentinel node Injection procedure;radioactive tracer for 38792 identification of sentinel node 120 Nuclear Medicine Rev Code SI Ind 2011 Action Code 0340, 034X S DESC 78580 0340, 034X S DESC 2012 Code Description Pulmonary perfusion imaging, particulate 78580 Pulmonary perfusion imaging (eg, particulate) 121 Nuclear Medicine SI Rev Code Ind 0340, 034X S 0340, 034X 0340, 034X S S 2012 Code Description Pulmonary perfusion imaging, particulate, with ventilation; single breath 78584 Pulmonary perfusion imaging, particulate, with ventilation; rebreathing and washout, with or without single breath 78585 2011 Action Code DEL DEL DEL 78586 0340, 034X S DEL 78587 0340, 034X S DEL 78588 Pulmonary ventilation imaging, aerosol; single projection Pulmonary ventilation imaging, aerosol; multiple projections (eg, anterior, posterior, lateral views) Pulmonary perfusion imaging, particulate, with ventilation imaging, aerosol, 1 or multiple projections 122 Nuclear Medicine Rev Code SI Ind 2011 Action Code 2012 Code Description 0340, 034X S DEL 78591 0340, 034X S DEL 78593 0340, 034X S DEL 78594 Pulmonary ventilation imaging, gaseous, single breath, single projection Pulmonary ventilation imaging, gaseous, with rebreathing and washout with or without single breath; single projection Pulmonary ventilation imaging, gaseous, with rebreathing and washout with or without single breath; multiple projections (eg, anterior, posterior, lateral views) 78596 Pulmonary quantitative differential function (ventilation/perfusion) study 0340, 034X S DEL 123 Nuclear Medicine SI Rev Code Ind 0340, 034X S 0340, 034X 0340, 034X S S 2011 Action Code 2012 Code Description NEW 78579 Pulmonary ventilation imaging (eg, aerosol or gas) DESC 78580 Pulmonary perfusion imaging (eg, particulate) NEW Pulmonary ventilation (eg, aerosol or gas) and 78582 perfusion imaging 0340, 034X S NEW 0340, 034X S NEW Quantitative differential pulmonary perfusion, 78597 including imaging when performed Quantitative differential pulmonary perfusion and ventilation (eg, aerosol or gas), including imaging 78598 when performed 124 Nuclear Medicine Rev Code 0343, 0636 0343, 0636 SI Ind G G 2011 Action Code DEL NEW C9406 2012 Code Description Iodine I-123 ioflupane, diagnostic, per study dose, up to 5 millicuries Iodine I-123 ioflupane, diagnostic, per study dose, A9584 up to 5 millicuries 125 Payment Changes for Radiopharmaceuticals A9582 Iodine I-123 iobenguane R 2012 SI e SI APC l N G A9583 Gadofosveset trisodium inj N HCPCS Short Descriptor Code G 2011 Payment 2636.16 12.65 126 Laboratory, Pathology and Blood Bank CPT and HCPCS code changes Molecular Diagnostics New CPT Codes Genetic Testing New CPT Codes Other Laboratory CPT Code Updates In 2012, 31% of coding changes reside in the Laboratory Chargemaster Laboratory CPT Changes- Immunology Rev SI 2011 2012 Code Ind Action Code Code Description 0300, 0302, 0309 A DESC 86703 Antibody; HIV-1 and HIV-2, single assay 0300, 0302, 0309 A DESC 86703 Antibody; HIV-1 and HIV-2, single result One CPT code to report a test for both HIV I and HIV II. 129 Laboratory CPT Changes- Pathology/Histology • In 2012, special stains have undergone descriptor revisions – Removal of personal identifications, e.g. Gridley Rev Code SI 2011 Ind Action Code 0310, 0312 X 0310, 0312 X 2012 Code Description Special stains; Group I for microorganisms (eg, Gridley, acid fast, methenamine silver), DESC 88312 including interpretation and report, each Special stain including interpretation and report; Group I for microorganisms (eg, acid DESC 88312 fast, methenamine silver) 130 Laboratory CPT Changes- Pathology/Histology Rev Code SI 2011 Ind Action Code 0310, 0312 X 0310, 0312 X 2012 Code Description Special stains; Group II, all other (eg, iron, trichrome), except immunocytochemistry and immunoperoxidase stains, including DESC 88313 interpretation and report, each Special stain including interpretation and report; Group II, all other (eg, iron, trichrome), except stain for microorganisms, stains for enzyme constituents or immunocytochemistry and DESC 88313 immunohistochemistry For determinative histochemistry to identify chemistry components, see 88313 131 Laboratory CPT Changes- Pathology/Histology Rev Code 0310, 0312 0310, 0312 SI 2011 Ind Action Code X DEL 88318 X Exist 88313 2012 Code Description Determinative histochemistry to identify chemical components (eg, copper, zinc) Special stain including interpretation and report; Group II, all other (eg, iron, trichrome), except stain for microorganisms, stains for enzyme constituents or immunocytochemistry and immunohistochemistry 132 Laboratory CPT Changes- Pathology/Histology Rev Code SI 2011 Ind Action Code 0310, 0312 X 0310, 0312 X 2012 Code Description Special stains; histochemical staining with frozen section(s), including interpretation and report (List separately in addition to code DESC 88314 for primary procedure) Special stain including interpretation and report; histochemical stain on frozen tissue block (List separately in addition to code for DESC 88314 primary procedure) 133 Laboratory CPT Changes- Pathology/Histology Rev Code 0310, 0312 0310, 0312 SI 2011 Ind Action Code X X 2012 Code Description Determinative histochemistry or cytochemistry DESC 88319 to identify enzyme constituents, each Special stains including interpretation and DESC 88319 report; Group III for enzyme constituents Deleted reference to histochemistry or cytochemistry in 2012.. Any time performing a special stain for enzyme constituents, reportable using CPT 88319. 134 Laboratory CPT Changes- Pathology/Histology Rev Code SI 2011 Ind Action Code 0310, 0311 X DEL 0310, 0311 X Exist 0310, 0311 X Exist 2012 Code Description Cytopathology, fluids, washings or brushings, except cervical or vaginal; smears and simple 88107 filter preparation with interpretation Cytopathology, fluids, washings or brushings, except cervical or vaginal; simple filter method 88106 with interpretation Cytopathology, fluids, washings or brushings, except cervical or vaginal; smears with 88104 interpretation 135 NEW Laboratory CPT Codes- Immunology Rev SI 2011 Code Ind Action Code 0300, 0302, 0309 A ADD 2012 Code Description 86386 Nuclear Matrix Protein 22 (NMP22), qualitative 136 NEW Laboratory CPT Codes- Bacteriology/Microbiology Rev Code 0300, 0306, 0309 SI 2011 Ind Action Code A ADD 2012 Code Description Infectious agent antigen detection by enzyme immunoassay technique, qualitative or semiquantitative, multiple-step method; HIV-1 antigen(s), with HIV-1 and HIV-2 antibodies, 87389 single result 137 Significant Laboratory Code Additions • New Molecular Codes – Phase I • Infectious disease agent DNA probes, direct and amplified – Moved from Chemistry section to the 87XXX Code Range – Phase II • Revisions were planned prior to 2010 – Codes were not unexpected or unannounced – Welcome by facilities that perform molecular diagnostics 138 Molecular Pathology • New Section between Urinalysis and Chemistry – New Terminology – New Definitions • Tier I Molecular Diagnostics • Tier II Molecular Pathology Procedures 139 140 141 Significant Laboratory Code Additions Molecular diagnostics o Genomics, analysis of genes o Genetic testings are encompassed in molecular diagnostics Protein aberrations that can manifest as disease Molecular pathology o Looking for variants of enzymes, factors or alleles responsible for impairment or disease 142 Molecular Diagnostics and Genetic Testing • Many techniques to determine if DNA sample has a mutation. Method of testing, when specified in a code, becomes a key coding term First Tier of Codes • Common genetic tests for uncommon genetic diseases • CPT 812XX – 813XX codes • Over 112 genetic tests for solid tumors and hematological cancers Second Tier of Codes • Molecular Pathology • As many as 1,000 tests are reported using one of nine Molecular Pathology CPT codes • CPT 81400-81408 143 Molecular Diagnostics and Genetic Testing • For 2012 new codes: – Eliminate code stacking • Explode codes • Multiple codes that reflect each step performed when doing genetic test or molecular diagnostic procedure – Cystic fibrosis test panel could have 5-9 separately reportable CPT codes • In 2012-one CPT code to report a single genetic assay 144 Molecular Diagnostics and Genetic Testing • Changes in 2012 are essentially moving away from the assignment of several nonspecific molecular codes to a single specific code – Less charge lines – Fewer explode panels • Molecular Pathology levels are similar to those in surgical pathology levels – Expand coding options for HLAS to include HLA-typing by genetic testing • Important for transplant, typing and matching 145 Molecular Diagnostics and Genetic Testing • If performing molecular diagnostic or genetic testing code updates: – Read preamble and parenthetical statements in Laboratory Section of 2012 CPT book – Become familiar with acronyms in new CPT code descriptions • Acronyms are standardized • Codes are alphabetized – Develop standardized chargemaster descriptions for new codes – Consider creating unique cost center for molecular/genetics • Some codes may be currently in Molecular, others in Chemistry – Review explode panels currently in use – Review use of genetic modifiers, Appendix I • Used primarily by independent laboratories, not by hospitals 146 Molecular Diagnostics and Genetic Testing • If performing molecular diagnostic or genetic testing code updates: • Work closely with Reference Lab when assigning codes • If performed in-house, may need to refer to test-kit inserts or laboratory procedure manuals – May not know if target is major or minor break point 147 Molecular Diagnostics and Genetic Testing • Currently Medicare is reimbursing only for Warfarin test – A pharmacogenetic test to determine how a patient metabolizes this specific drug • Coverage of these new codes by Medicare and payers should not be assumed – Medicare has met to determine the applicability of these new codes, medical necessity and how to address them in the laboratory fee schedule 148 Molecular Diagnostics and Genetic Testing References: – International Society of Genetic Geneology • http://www.ISOGG.org – Glossary of DNA terms – National Cancer Institute • http://www.CANCER.gov – Tutorials • Molecular diagnostics and is straightforward (found under cancer topics) 149 Molecular Diagnostics and Genetic Testing • Tier I – Molecular Pathology – Codes 81200-81350 • Gene-specific and genomic procedures • Tier 2 – Molecular Pathology – Codes 81400-81408 • Medically useful procedures that are generally performed in lower volumes than Tier I procedures • If the analyte tested is not listed under one of the Tier 2 codes or is not represented by a Tier code, use the appropriate methodology codes in the 83890-83914 and 88384-88386 series 150 Molecular Diagnostics and Genetic Testing-Tier 1 Rev Code 0300, 0301, 0309 0300, 0301, 0309 SI 2011 Ind Action Code B ADD B ADD 2012 Code Description ASPA (aspartoacylase) (eg, Canavan disease) gene analysis, common variants (eg, E285A, 81200 Y231X) BCKDHB (branched-chain keto acid dehydrogenase E1, beta polypeptide) (eg, Maple syrup urine disease) gene analysis, common 81205 variants (eg, R183P, G278S, E422X) 151 Molecular Diagnostics and Genetic Testing-Tier 1 Rev Code 0300, 0301, 0309 0300, 0301, 0309 0300, 0301, 0309 SI 2011 Ind Action Code B ADD B ADD B ADD 2012 Code Description BCR/ABL1 (t(9;22)) (eg, chronic myelogenous leukemia) translocation analysis; major 81206 breakpoint, qualitative or quantitative BCR/ABL1 (t(9;22)) (eg, chronic myelogenous leukemia) translocation analysis; minor 81207 breakpoint, qualitative or quantitative BCR/ABL1 (t(9;22)) (eg, chronic myelogenous leukemia) translocation analysis; other 81208 breakpoint, qualitative or quantitative 152 Molecular Diagnostics and Genetic Testing-Tier 1 Rev Code 0300, 0301, 0309 0300, 0301, 0309 SI 2011 Ind Action Code B ADD B ADD 2012 Code Description BLM (Bloom syndrome, RecQ helicase-like) (eg, Bloom syndrome) gene analysis, 2281del6ins7 81209 variant BRAF (v-raf murine sarcoma viral oncogene homolog B1) (eg, colon cancer), gene analysis, 81210 V600E variant 153 Molecular Diagnostics and Genetic Testing-Tier 1 Rev Code 0300, 0301, 0309 0300, 0301, 0309 0300, 0301, 0309 SI 2011 Ind Action Code B ADD B ADD B ADD 2012 Code Description BRCA1, BRCA2 (breast cancer 1 and 2) (eg, hereditary breast and ovarian cancer) gene analysis; full sequence analysis and common duplication/deletion variants in BRCA1 (ie, exon 13 del 3.835kb, exon 13 dup 6kb, exon 14-20 del 81211 26kb, exon 22 del 510bp, exon 8-9 del 7.1kb) BRCA1, BRCA2 (breast cancer 1 and 2) (eg, hereditary breast and ovarian cancer) gene 81212 analysis; 185delAG, 5385insC, 674delT variants BRCA1, BRCA2 (breast cancer 1 and 2) (eg, hereditary breast and ovarian cancer) gene analysis; uncommon duplication/deletion 81213 variants 154 Molecular Diagnostics and Genetic Testing-Tier 1 Rev Code 0300, 0301, 0309 0300, 0301, 0309 SI 2011 Ind Action Code B ADD B ADD 2012 Code Description BRCA1 (breast cancer 1) (eg, hereditary breast and ovarian cancer) gene analysis; full sequence analysis and common duplication/deletion variants (ie, exon 13 del 3.835kb, exon 13 dup 6kb, exon 14-20 del 26kb, exon 22 del 510kb, 81214 exon 8-9 del 7.1kb) BRCA1 (breast cancer 1) (eg, hereditary breast and ovarian cancer) gene analysis; known 81215 familial variant 155 Molecular Diagnostics and Genetic Testing-Tier 1 Rev Code 0300, 0301, 0309 0300, 0301, 0309 SI 2011 Ind Action Code B ADD B ADD 2012 Code Description BRCA2 (breast cancer 2) (eg, hereditary breast and ovarian cancer) gene analysis; full sequence 81216 analysis BRCA2 (breast cancer 2) (eg, hereditary breast and ovarian cancer) gene analysis; known 81217 familial variant 156 Molecular Diagnostics and Genetic Testing-Tier 1 Rev Code 0300, 0301, 0309 0300, 0301, 0309 0300, 0301, 0309 0300, 0301, 0309 0300, 0301, 0309 SI Ind Action B ADD B ADD B ADD B ADD B ADD 2011 Code 2012 Code Description CFTR (cystic fibrosis transmembrane conductance regulator) (eg, cystic fibrosis) gene analysis; common variants (eg, ACMG/ACOG 81220 guidelines) CFTR (cystic fibrosis transmembrane conductance regulator) (eg, cystic fibrosis) gene 81221 analysis; known familial variants CFTR (cystic fibrosis transmembrane conductance regulator) (eg, cystic fibrosis) gene 81222 analysis; duplication/deletion variants CFTR (cystic fibrosis transmembrane conductance regulator) (eg, cystic fibrosis) gene 81223 analysis; full gene sequence CFTR (cystic fibrosis transmembrane conductance regulator) (eg, cystic fibrosis) gene analysis; intron 8 poly-T analysis (eg, male 81224 infertility) 157 Molecular Diagnostics and Genetic Testing-Tier 1 Rev Code 0300, 0301, 0309 B ADD 0300, 0301, 0309 B ADD 2012 Code Description CYP2C19 (cytochrome P450, family 2, subfamily C, polypeptide 19) (eg, drug metabolism), gene analysis, common variants 81225 (eg, *2, *3, *4, *8, *17) CYP2D6 (cytochrome P450, family 2, subfamily D, polypeptide 6) (eg, drug metabolism), gene analysis, common variants (eg, *2, *3, *4, *5, *6, *9, *10, *17, *19, *29, *35, *41, *1XN, *2XN, 81226 *4XN) ADD CYP2C9 (cytochrome P450, family 2, subfamily C, polypeptide 9) (eg, drug metabolism), gene 81227 analysis, common variants (eg, *2, *3, *5, *6) 0300, 0301, 0309 SI 2011 Ind Action Code B Drug Metabolism testing 158 Molecular Diagnostics and Genetic Testing-Tier 1 Rev Code SI 2011 Ind Action Code 0300, 0301, 0309 B ADD 0300, 0301, 0309 B ADD 2012 Code Description Cytogenomic constitutional (genome-wide) microarray analysis; interrogation of genomic regions for copy number variants (eg, Bacterial Artificial Chromosome [BAC] or oligo-based comparative genomic hybridization [CGH] 81228 microarray analysis) Cytogenomic constitutional (genome-wide) microarray analysis; interrogation of genomic regions for copy number and single nucleotide polymorphism (SNP) variants for chromosomal 81229 abnormalities 159 Molecular Diagnostics and Genetic Testing-Tier 1 Rev Code 0300, 0301, 0309 0300, 0301, 0309 0300, 0301, 0309 SI 2011 Ind Action Code B ADD B ADD B ADD 2012 Code Description F2 (prothrombin, coagulation factor II) (eg, hereditary hypercoagulability) gene analysis, 81240 20210G>A variant F5 (coagulation Factor V) (eg, hereditary 81241 hypercoagulability) gene analysis, Leiden variant FANCC (Fanconi anemia, complementation group C) (eg, Fanconi anemia, type C) gene 81242 analysis, common variant (eg, IVS4+4A>T) 160 Molecular Diagnostics and Genetic Testing-Tier 1 Rev Code SI 2011 Ind Action Code 0300, 0301, 0309 B ADD 0300, 0301, 0309 B ADD 0300, 0301, 0309 B ADD 2012 Code Description FMR1 (Fragile X mental retardation 1) (eg, fragile X mental retardation) gene analysis; evaluation 81243 to detect abnormal (eg, expanded) alleles FMR1 (Fragile X mental retardation 1) (eg, fragile X mental retardation) gene analysis; characterization of alleles (eg, expanded size 81244 and methylation status) FLT3 (fms-related tyrosine kinase 3) (eg, acute myeloid leukemia), gene analysis, internal tandem duplication (ITD) variants (ie, exons 14, 81245 15) 161 Molecular Diagnostics and Genetic Testing-Tier 1 Rev Code 0300, 0301, 0309 0300, 0301, 0309 0300, 0301, 0309 SI 2011 Ind Action Code B ADD B ADD B ADD 2012 Code Description G6PC (glucose-6-phosphatase, catalytic subunit) (eg, Glycogen storage disease, Type 1a, von Gierke disease) gene analysis, common 81250 variants (eg, R83C, Q347X) GBA (glucosidase, beta acid) (eg, Gaucher disease) gene analysis, common variants (eg, 81251 N370S, B4GG, L444P, IVS2+1G>A) HEXA (hexosaminidase A [alpha polypeptide]) (eg, Tay-Sachs disease) gene analysis, common variants (eg, 1278insTATC, 1421+G>C, 81255 G269S) 162 Molecular Diagnostics and Genetic Testing-Tier 1 Rev Code 0300, 0301, 0309 SI 2011 Ind Action Code B ADD 0300, 0301, 0309 B ADD 0300, 0301, 0309 B ADD 2012 Code Description HFE (hemochromatosis) (eg, hereditary hemochromatosis) gene analysis, common 81256 variants (eg, C282Y, H63D) HBA1/HBA2 (alpha globin 1 and alpha globin 2) (eg, alpha thalassema, Hb Bart hydrops fetalis syndrome, HbH disease), gene analysis, for common deletions or variant (eg, Southeast Asian, Thai, Filipino, Mediterranean, alpha3.7, 81257 alpha4.2, alpha20.5, and Constant Spring) IKBKAP (inhibitor of kappa light polypeptide gene enhancer in B-cells, kinase complexassociated protein) (eg, familial dysautonomia) gene analysis, common variants (eg, 81260 2507+6T>C, R696P) 163 Molecular Diagnostics and Genetic Testing-Tier 1 Rev Code 0300, 0301, 0309 0300, 0301, 0309 0300, 0301, 0309 0300, 0301, 0309 SI 2011 Ind Action Code B ADD B ADD B ADD B ADD 2012 Code Description IGH@ (Immunoglobulin heavy chain locus) (eg, leukemias and lymphomas, B-cell), gene rearrangement analysis to detect abnormal clonal population(s); amplified methodology (eg, 81261 polymerase chain reaction) IGH@ (Immunoglobulin heavy chain locus) (eg, leukemias and lymphomas, B-cell), gene rearrangement analysis to detect abnormal clonal population(s); direct probe methodology 81262 (eg, Southern blot) IGH@ (Immunoglobulin heavy chain locus) (eg, leukemia and lymphoma, B-cell), variable region 81263 somatic mutation analysis IGK@ (Immunoglobulin kappa light chain locus) (eg, leukemia and lymphoma, B-cell) gene rearrangement analysis, evaluation to detect 81264 abnormal clonal population(s) 164 Molecular Diagnostics and Genetic Testing-Tier 1 Rev Code SI 2011 Ind Action Code 0300, 0301, 0309 B ADD 0300, 0301, 0309 B ADD 2012 Code Description Comparative analysis using Short Tandem Repeat (STR) markers; patient and comparative specimen (eg, pre-transplant recipient and donor germline testing, post-transplant nonhematopoietic recipient germline (eg, buccal swab orother germline tissue sample) and donor testing, twin zygosity testing, or maternal cll 81265 contamination of fetal cells) Comparative analysis using Short Tandem Repeat (STR) markers; each additional specimen (eg, additional cord blood donor, additional fetal samples from different cultures, or additional zygosity in multiple birth pregnancies) (List separately in addition to code 81266 for primary procedure) 165 Molecular Diagnostics and Genetic Testing-Tier 1 Rev Code SI 2011 Ind Action Code 0300, 0301, 0309 B ADD 0300, 0301, 0309 B ADD 2012 Code Description Chimerism (engraftment) analysis, post transplantation specimen (eg, hematopoietic stem cell), includes comparison to previously performed baseline analysis; without cell 81267 selection Chimerism (engraftment) analysis, post transplantation specimen (eg, hematopoietic stem cell), includes comparison to previously performed baseline analysis; with cell selection 81268 (eg, CD3, CD33), each cell type Comparing a specimen today with a previous specimen to see if there are two types of DNA---used to check if stem cell transplant has taken 166 Molecular Diagnostics and Genetic Testing-Tier 1 Rev Code 0300, 0301, 0309 0300, 0301, 0309 SI 2011 Ind Action Code B ADD B ADD 2012 Code Description JAK2 (Janus kinase 2) (eg, myeloproliferative disorder) gene analysis, p.Val617Phe (V617F) 81270 variant KRAS (v-Ki-ras2 Kirsten rat sarcoma viral oncogene) (eg, carcinoma) gene analysis, 81275 variants in codons 12 and 13 167 Molecular Diagnostics and Genetic Testing-Tier 1 Rev Code 0300, 0301, 0309 B ADD 0300, 0301, 0309 B ADD 2012 Code Description Long QT syndrome gene analyses (eg, KCNQ1, KCNH2, SCN5A, KCNE1, KCNE2, KCNJ2, CACNA1C, CAV3, SCN4B, AKAP, SNTA1, and 81280 ANK2); full sequence analysis Long QT syndrome gene analyses (eg, KCNQ1, KCNH2, SCN5A, KCNE1, KCNE2, KCNJ2, CACNA1C, CAV3, SCN4B, AKAP, SNTA1, and 81281 ANK2); known familial sequence variant ADD Long QT syndrome gene analyses (eg, KCNQ1, KCNH2, SCN5A, KCNE1, KCNE2, KCNJ2, CACNA1C, CAV3, SCN4B, AKAP, SNTA1, and 81282 ANK2); duplication/deletion variants 0300, 0301, 0309 SI 2011 Ind Action Code B 168 Molecular Diagnostics and Genetic Testing-Tier 1 Rev Code 0300, 0301, 0309 0300, 0301, 0309 SI 2011 Ind Action Code B ADD B ADD 2012 Code Description MCOLN1 (mucolipin 1) (eg, Mucolipidosis, type (V) gene analysis, common variants (eg, IVS381290 2A>G, del6.4kb) MTHFR (5, 10-methylenetetrahydrofalate reductase) (eg, hereditary hypercoagulability) gene analysis, common variants (eg, 677T, 81291 1298C) 169 Molecular Diagnostics and Genetic Testing-Tier 1 Rev Code SI 2011 Ind Action Code 0300, 0301, 0309 B ADD 0300, 0301, 0309 B ADD 0300, 0301, 0309 B ADD 2012 Code Description MLH1 (mutL homolog 1, colon cancer, nonpolyposis type 2) (eg, hereditary nonpolyposis colorectal cancer, Lynch syndrome) 81292 gene analysis; full sequence analysis MLH1 (mutL homolog 1, colon cancer, nonpolyposis type 2) (eg, hereditary nonpolyposis colorectal cancer, Lynch syndrome) 81293 gene analysis; known familial variants MLH1 (mutL homolog 1, colon cancer, nonpolyposis type 2) (eg, hereditary nonpolyposis colorectal cancer, Lynch syndrome) 81294 gene analysis; duplication/deletion variants 170 Molecular Diagnostics and Genetic Testing-Tier 1 Rev Code 2011 SI Ind Action Code 0300, 0301, 0309 B ADD 0300, 0301, 0309 B ADD 0300, 0301, 0309 B ADD 2012 Code Description MSH2 (mutS homolog 2, colon cancer, nonpolyposis type 1) (eg, hereditary nonpolyposis colorectal cancer, Lynch syndrome) 81295 gene analysis; full sequence analysis MSH2 (mutS homolog 2, colon cancer, nonpolyposis type 1) (eg, hereditary nonpolyposis colorectal cancer, Lynch syndrome) 81296 gene analysis; known familial variants MSH2 (mutS homolog 2, colon cancer, nonpolyposis type 1) (eg, hereditary nonpolyposis colorectal cancer, Lynch syndrome) 81297 gene analysis; duplication/deletion variants 171 Molecular Diagnostics and Genetic Testing-Tier 1 Rev Code 0300, 0301, 0309 0300, 0301, 0309 0300, 0301, 0309 SI 2011 Ind Action Code B ADD B ADD B ADD 2012 Code Description MSH6 (mutS homolog 6 [E. coli]) (eg, hereditary non-polyposis colorectal cancer, Lynch 81298 syndrome) gene analysis; full sequence analysis MSH6 (mutS homolog 6 [E. coli]) (eg, hereditary non-polyposis colorectal cancer, Lynch 81299 syndrome) gene analysis; known familial variants MSH6 (mutS homolog 6 [E. coli]) (eg, hereditary non-polyposis colorectal cancer, Lynch syndrome) gene analysis; duplication/deletion 81300 variants 172 Molecular Diagnostics and Genetic Testing-Tier 1 Rev Code 0300, 0301, 0309 SI 2011 Ind Action Code B ADD 2012 Code Description Microsatellite instabiilty analysis (eg, hereditary non-polyposis colorectal cancer, Lynch syndrome) or markers for mismatch repair deficiency (eg, BAT25, BAT26), includes comparison of neoplastic and normal tissue, if 81301 performed 173 Molecular Diagnostics and Genetic Testing-Tier 1 Rev Code 0300, 0301, 0309 0300, 0301, 0309 0300, 0301, 0309 SI 2011 Ind Action Code B ADD B ADD B ADD 2012 Code Description MECP2 (methyl CpG binding protein 2) (eg, Rett 81302 syndrome) gene analysis; full sequence analysis MECP2 (methyl CpG binding protein 2) (eg, Rett 81303 syndrome) gene analysis; known familial variant MECP2 (methyl CpG binding protein 2) (eg, Rett syndrome) gene analysis; duplication/deletion 81304 variants 174 Molecular Diagnostics and Genetic Testing-Tier 1 Rev Code 0300, 0301, 0309 SI 2011 Ind Action Code B ADD 2012 Code Description NPM1 (nucleophosmin) (eg, acute myeloid 81310 leukemia) gene analysis, exon 12 variants 175 Molecular Diagnostics and Genetic Testing-Tier 1 Rev Code SI 2011 Ind Action Code 0300, 0301, 0309 B ADD 0300, 0301, 0309 B ADD 2012 Code Description PML/RARalpha, (t(15; 17)), (promyelocytic leukemia/retinoic acid receptor alpha) (eg, promyelocytic leukemia) translocation analysis; common breakpoints (eg, intron 3 and intron 6), 81315 qualitative or quanitative PML/RARalpha, (t(15; 17)), (promyelocytic leukemia/retinoic acid receptor alpha) (eg, promyelocytic leukemia) translocation analysis; single breakpoint (eg, intron 3, intron 6 or exon 81316 6), qualitative or quantitative 176 Molecular Diagnostics and Genetic Testing-Tier 1 Rev Code SI 2011 Ind Action Code 0300, 0301, 0309 B ADD 0300, 0301, 0309 B ADD 0300, 0301, 0309 B ADD 2012 Code Description PMS2 (postmeiotic segregation increased 2 [?S. cerevisiae]) (eg, hereditary non-polyposis colorectal cancer, Lynch syndrome) gene 81317 analysis; full sequence analysis PMS2 (postmeiotic segregation increased 2 [?S. cerevisiae]) (eg, hereditary non-polyposis colorectal cancer, Lynch syndrome) gene 81318 analysis; known familial variants PMS2 (postmeiotic segregation increased 2 [?S. cerevisiae]) (eg, hereditary non-polyposis colorectal cancer, Lynch syndrome) gene 81319 analysis; duplication/deletion variants 177 Molecular Diagnostics and Genetic Testing-Tier 1 Rev Code 0300, 0301, 0309 SI 2011 Ind Action Code B ADD 0300, 0301, 0309 B ADD 0300, 0301, 0309 B ADD 2012 Code Description SMPD1 (sphingomyel phosphodiesterase 1, acid lysosomal) (eg, Niemann-Pick disease, Type A) gene analysis, common variants (eg, R496L, 81330 L302P, fsP330) SNRPN/UBE3A (small nuclear ribonucleoprotein polypeptide N and ubiquitin protein ligase E3A) (eg, Prader-Will syndrome and/or Angelman 81331 syndrome), methylation analysis SERPINA1 (serpin peptidase inhibitor, clade A, alpha-1 antiproteinase, antitrypsin, member 1) (eg, alpha-1-antitrypsin deficiency), gene 81332 analysis, common variants (eg, *S and *Z) 178 Molecular Diagnostics and Genetic Testing-Tier 1 Rev Code SI 2011 Ind Action Code 0300, 0301, 0309 B ADD 0300, 0301, 0309 B ADD 2012 Code Description TRB@ (T cell antigen receptor, beta) (eg, leukemia and lymphoma), gene rearrangement analysis to detect abnormal clonal population(s); using amplification methodology (eg, polymerase 81340 chain reaction) TRB@ (T cell antigen receptor, beta) (eg, leukemia and lymphoma), gene rearrangement analysis to detect abnormal clonal population(s); using direct probe methodology (eg, Southern 81341 blot) 179 Molecular Diagnostics and Genetic Testing-Tier 1 Rev Code 0300, 0301, 0309 B ADD 0300, 0301, 0309 B ADD 2012 Code Description TRG@ (T cell antigen receptor, gamma) (eg, leukemia and lymphoma), gene rearrangement analysis, evaluation to detect abnormla clonal 81342 population(s) UGT1A1 (UDP glucuronosyltransferase 1 family, polypeptide A1) (eg, irinotecan metabolism), gene analysis, common variants (eg, *28, *36, 81350 *37) ADD VKORC1 (vitamin K epoxide reductase complex, subunit 1) (eg, warfarin metabolism), gene 81355 analysis, common variants (eg, -16397/3673) 0300, 0301, 0309 SI 2011 Ind Action Code B 180 Molecular Diagnostics and Genetic Testing-Tier 1 • Human Leukocyte Antigen – HLA Testing • Codes 81370-81383 – Assess recipient and potential donor compatibility for solid organ or hematopoetic stem cell transplantation • HLA alleles and allele groups associated with specific diseases and response to drug therapy – Each HLA gene typically has multiple variant alleles or allele groups that can be identified for grouping 181 Molecular Diagnostics and Genetic Testing-Tier 1 Rev Code 0300, 0301, 0309 0300, 0301, 0309 0300, 0301, 0309 SI 2011 Ind Action Code B ADD B ADD 2012 Code Description HLA Class I and II typing, low resolution (eg, antigen equivalents); HLA-A, -B, -C, -DRB1/3/4/5 81370 and -DQB1 HLA Class I and II typing, low resolution (eg, antigen equivalents); HLA-A, -B and -DRB1/3/4/5 81371 (eg, verification typing) ADD HLA Class I typing, low resolution (eg, antigen 81372 equivalents); complete (ie HLA-A, -B and -C) B 182 Molecular Diagnostics and Genetic Testing-Tier 1 Rev Code 0300, 0301, 0309 0300, 0301, 0309 SI 2011 Ind Action Code B ADD B ADD 2012 Code Description HLA Class I typing, low resolution (eg, antigen equivalents); one locus (eg, HLA-A, -B, or -C), 81373 each HLA Class I typing, low resolution (eg, antigen equivalents); one antigen equivalent (eg, B*27), 81374 each 183 Molecular Diagnostics and Genetic Testing-Tier 1 Rev Code 0300, 0301, 0309 0300, 0301, 0309 0300, 0301, 0309 SI 2011 Ind Action Code 2012 Code Description B ADD B ADD HLA Class II typing, low resolution (eg, antigen 81375 equivalents); HLA-DRB1/3/4/5 and -DQB1 HLA Class II typing, low resolution (eg, antigen equivalents); one locus (eg, HLA-DRB1/3/4/5, 81376 DQB1, -DQA1, -DPB1, or -DPA1), each ADD HLA Class II typing, low resolution (eg, antigen 81377 equivalents); one antigen equivalent, each B 184 Molecular Diagnostics and Genetic Testing-Tier 1 Rev Code 0300, 0301, 0309 0300, 0301, 0309 0300, 0301, 0309 0300, 0301, 0309 SI 2011 Ind Action Code B ADD B ADD B ADD B ADD 2012 Code Description HLA Class I and II typing, high resolution (ie, alleles or allele groups), HLA-A, -B, -C, and 81378 DRB1 HLA Class I typing, high resolution (ie, alleles or 81379 allele groups); complete (ie, HLA-A, -B and -C); HLA Class I typing, high resolution (ie, alleles or allele groups); one locus (eg, HLA-A, -B, or -C), 81380 each HLA Class I typing, high resolution (ie, alleles or allele groups); one allele or allele group (eg, 81381 B*57:01P), each 185 Molecular Diagnostics and Genetic Testing-Tier 1 Rev Code 0300, 0301, 0309 0300, 0301, 0309 SI 2011 Ind Action Code B B ADD ADD 2012 Code Description HLA Class II typing, high resolution (ie, alleles or allele groups); one locus (eg, HLA-DRB1, DRB3, -DRB4, -DRB5, -DQB1, -DQA1, -DPB1, 81382 or -DPA1), each HLA Class II typing, high resolution (ie, alleles or allele groups); one allele or allele group (eg, HLA81383 DQB1 *06:02P), each 186 Molecular Pathology-Tier 2 Molecular Pathology • Looking for variants of enzymes, alleles that are responsible for impairment or disease – Codes assigned based on key indicators in descriptors by number of SNPS, variants or exons • As SNPS go up, variants go up and the analysis performed becomes more complex – First level describes an analysis of a single SNP using a fairly straightforward technique – Level 9 describes an analysis of more than 50 exons in a single gene that encompasses whole genome sequency test 188 Molecular Pathology-Tier 2 Rev Code SI 2011 Ind Action Code 0300, 0301, 0309 B ADD 0300, 0301, 0309 B ADD 2012 Code Description Molecular pathology procedure, Level 1 (eg, identification of single germline variant [eg, SNP] by techniques such as restriction enzyme 81400 digestion or melt curve analysis) Molecular pathology procedure, Level 2 (eg, 2-10 SNPs, 1 methylated variant, or 1 somatic variant [typically using nonsequencing target variant analysis], or detection of a dynamic mutation 81401 disorder/triplet repeat) 189 Molecular Pathology -Tier 2 Rev Code SI 2011 Ind Action Code 0300, 0301, 0309 B ADD 0300, 0301, 0309 B ADD 2012 Code Description Molecular pathology procedure, Level 3 (eg, >10 SNPs, 2-10 methylated variants, or 2-10 somatic variants [typically using non-sequencing target variant analysis], immunoglobulin and T-cell receptor gene rearrangements, 81402 duplication/deletion variants 1 exon) Molecular pathology procedure, Level 4 (eg, analysis of single exon by DNA sequence analysis, analysis of >10 amplicons using multiplex PCR in 2 or more independent reactions, mutation scanning or 81403 duplication/deletion variants of 2-5 exons) 190 Molecular Pathology-Tier 2 Rev Code SI 2011 Ind Action Code 0300, 0301, 0309 B ADD 0300, 0301, 0309 B ADD 2012 Code Description Molecular pathology procedure, Level 5 (eg, analysis of 2-5 exons by DNA sequence analysis, mutation scanning or duplication/deletion variants of 6-10 exons, or characterization of a dynamic mutation 81404 disorder/triplet repeat by Southern blot analysis) Molecular pathology procedure, Level 6 (eg, analysis of 6-10 exons by DNA sequence analysis, mutation scanning or 81405 duplication/deletion variants of 11-25 exons) 191 Molecular Pathology-Tier 2 Rev Code SI 2011 Ind Action Code 0300, 0301, 0309 B ADD 0300, 0301, 0309 B ADD 2012 Code Description Molecular pathology procedure, Level 7 (eg, analysis of 11-25 exons by DNA sequence analysis, mutation scanning or duplication/deletion variants of 26-50 exons, 81406 cytogenomic array analysis for neoplasia) Molecular pathology procedure, Level 8 (eg, analysis of 26-50 exons by DNA sequence analysis, mutation scanning or duplication/deletion variants of > 50 exons, sequence analysis of multiple genes on one 81407 platform) 192 Molecular Pathology-Tier 2 Rev Code 0300, 0301, 0309 SI 2011 Ind Action Code B ADD 2012 Code Description Molecular pathology procedure, Level 9 (eg, analysis of > 50 exons in a single gene by DNA 81408 sequence analysis) 193 Other Laboratory Testing Rev Code SI 2011 Ind Action Code 0300, 0309, X ADD 0300, 0309, X ADD 2012 Code Description Cell enumeration using immunologic selection and identification in fluid specimen (eg, 0279T circulating tumor cells in blood); Cell enumeration using immunologic selection and identification in fluid specimen (eg, circulating tumor cells in blood); interpretation 0280T and report For cell enumeration using immunologic selection and identification in fluid specimen (eg, circulating tumor cells in blood) 194 Anesthesia Services No CPT and HCPCS code changes Infusion Services CPT and HCPCS code changes Infusion Services For both facility and physician reporting: • Initial – Only one initial code should be reported unless the protocol or patient’s condition requires two separate IV sites be utilized • Reportable with modifier -59 • Sequential – An infusion or IV push of a new substance or drug following a primary or initial service • Concurrent – Infusion of a new substance or drug infused at the same time as another substance or drug • Hydration may not be reported concurrently with any other service 197 Infusion Services • When reporting codes for which infusion time is a factor – Use the actual time over which the infusion is administered – Injections given before and after midnight may be both reported as an initial since they were not continuous…… – For continuous services that last beyond midnight, use the date in which the service began and report the total units of time provided continuously. – A “keep open” infusion of any time is not separately reported 198 Infusion Services • Some chemotherapeutic agents and other therapeutic agents require pre- and/or post-hydration to be given in order to avoid specific toxicities – Minimum 31 minutes duration of hydration required – Not used for the purpose of intravenous fluid is to “keep open” an IV line prior to or subsequent to a therapeutic infusion or as a freeflowing IV during chemotherapy or other therapeutic infusion 199 Infusion Therapy Services Rev SI Code Ind Action 0260, Var S DESC 0260, Var S DESC 2011 Code 2012 Code Description Intravenous infusion, for therapy, prophylaxis, or diagnosis (specify substance or drug); additional sequential infusion, up to 1 hour (List separately in 96367 addition to code for primary procedure) Intravenous infusion, for therapy, prophylaxis, or diagnosis (specify substance or drug); additional sequential infusion of a new drug/substance, up to 1 hour (List separately in addition to code for 96367 primary procedure) 200 Payment Comparison October 2011 to 2012 HCPCS Code 96366 96371 96372 90471 G0010 Short Descriptor Ther/proph/diag iv inf addon Sc ther infusion reset pump Ther/proph/diag inj sc/im Immunization admin Admin hepatitis b vaccine CI CH CH CH CH CH S I S S S S S APC 0437 0437 0437 0437 0437 2012 Payment Rate $34.81 $34.81 $34.81 $34.81 $34.81 S S S S S 2011 Pay Payment Rate Diff. 26.35 8.46 26.35 8.46 26.35 8.46 26.35 8.46 26.35 8.46 201 Behavioral Health Services CPT and HCPCS code changes Behavioral Health Services Rev Code 0510, 0513, 0920 0510, 0513, 0920 SI 2011 Ind Action Code S S 2012 Code Description Therapeutic repetitive transcranial magnetic DESC 90867 stimulation treatment; planning Therapeutic repetitive transcranial magnetic stimulation (TMS) treatment; initial, including cortical mapping, motor threshold determination, DESC 90867 delivery and management 203 Behavioral Health Services Common side effects and adverse health problems associated with TMS include: -- Headache -- Lightheadedness -- Discomfort from noise during treatment -- Pain at the site of stimulation -- Tingling of facial muscles -- Mild spasms or contractions of facial muscles 204 Behavioral Health Services Rev Code 0510, 0513, 0920 0510, 0513, 0920 Rev 0510, 0513, 0920 2012 Code Description Therapeutic repetitive transcranial magnetic stimulation treatment; delivery and management, per session DESC 90868 Therapeutic repetitive transcranial magnetic stimulation (TMS) treatment; subsequent delivery and DESC Description per session 2012 management, Action 2011 90868 Therapeutic repetitive transcranial magnetic stimulation (TMS) treatment; subsequent motor threshold re-determination with delivery and 90869 management ADD 2011 SI Ind Action Code S S SI S 205 Behavioral Health Services Rev Code 0510, 0513, 0920 2012 Code Description Therapeutic repetitive transcranial magnetic stimulation treatment delivery and management per session; 0161T Note: Listed as deleted in 2012 but actually code was deleted in 2011, reportable using 90867, 90868 2011 SI Ind Action Code DEL 206 Behavioral Health Services Rev Code 0918 0918 SI 2011 Ind Action Code 2012 Code Description Developmental testing; limited (eg, Developmental Screening Test II, Early Language Milestone Q3 DESC 96110 Screen), with interpretation and report Developmental screening, with interpretation and Q3 DESC 96110 report, per standardized instrument form 207 Behavioral Health Services Rev Code 0918 0918 SI 2011 Ind Action Code 2012 Code Description Developmental testing; extended (includes assessment of motor, language, social, adaptive and/or cognitive functioning by standardized developmental instruments) with interpretation and Q3 DESC 96111 report Developmental testing, (includes assessment of motor, language, social, adaptive, and/or cognitive functioning by standardized developmental Q3 DESC 96111 instruments) with interpretation and report 208 Behavioral Health Services Rev Code 051X SI 2011 Ind Action Code B NEW 2012 Code Description Development testing, with interpretation and report, G0451 per standardized instrument form 209 Pulmonary and Respiratory Therapy Services CPT and HCPCS code changes Pulmonary and Respiratory Services • New instructional notes and definitions: – Spirometry – Vital capacity – Flow-volume loop – Plethysmography – Nitrogen washout or helium dilution – Impulse oscillometry – Diffusion capacity • Parenthetical notes provide additional guidance on codes reportable or not reportable in combination 211 Pulmonary/Respiratory Therapy Services Rev Code 0460, 0480, 092X 0460, 0480, 092X 0460, 0480, 092X 0460, 0480, 092X 0460, 0480, 092X SI Ind B X B X X Action DEL DEL 2011 Code 2012 Code Description 93720 Plethysmography, total body; with interpretation and report 93721 Plethysmography, total body; tracing only, without interpretation and report Plethysmography, total body; DEL 93722 interpretation and report only Plethysmography for determination of lung volumes and, when performed, airway NEW 94726 resistance Pulmonary compliance study (eg, plethysmography, volume and pressure Existing 94750 measurements) 212 Pulmonary/Respiratory Therapy Services Rev Code 0460, 0480, 092X 0460, 0480, 092X 0460, 0480, 092X 0460, 0480, 092X SI Ind 2011 Code Action X DEL 2012 Code Description Functional residual capacity or residual volume: helium method, nitrogen open 94240 circuit method, or other method X DEL 94260 X NEW X NEW Thoracic gas volume Plethysmography for determination of lung volumes and, when performed, airway 94726 resistance Gas dilution or washout for determination of lung volumes and, when performed, distribution of ventilation and closing 94727 volumes 213 Pulmonary/Respiratory Therapy Services Rev Code 0460, 0480, 092X 0460, 0480, 092X 0460, 0480, 092X SI Ind Action X DEL X NEW X NEW 2011 Code 2012 Code Description Determination of maldistribution of inspired gas: multiple breath nitrogen washout curve including alveolar nitrogen or helium 94350 equilibration time Plethysmography for determination of lung volumes and, when performed, airway 94726 resistance Gas dilution or washout for determination of lung volumes and, when performed, distribution of ventilation and closing 94727 volumes 214 Pulmonary/Respiratory Therapy Services Rev Code 0460, 092X 0460, 0480, 092X 0460, 0480, 092X SI Ind 2011 Code Action X DEL X NEW 2012 Code Description Determination of resistance to airflow, 94360 oscillatory or plethysmographic methods Plethysmography for determination of lung volumes and, when performed, airway 94726 resistance X NEW 94728 Airway resistance by impulse oscillometry 215 Pulmonary/Respiratory Therapy Services Rev Code 0460, 092X 0460, 0480, 092X 0460, 0480, 092X SI Ind Action X DEL X NEW X NEW 2011 Code 2012 Code Description Determination of airway closing volume, 94370 single breath tests Plethysmography for determination of lung volumes and, when performed, airway 94726 resistance Gas dilution or washout for determination of lung volumes and, when performed, distribution of ventilation and closing 94727 volumes 216 Pulmonary/Respiratory Therapy Services Rev Code 0460, 092X 0460, 092X 0460, 092X SI Ind 2011 Code Action X DEL 2012 Code Description Carbon monoxide diffusing capacity (eg, 94720 single breath, steady state) X DEL 94725 X NEW Membrane diffusion capacity Diffusing capacity (eg, carbon monoxide, membrane) (List separately in addition to 94729 code for primaryprocedure) 217 Respiratory Rev SI Code Ind Action 0920, 092X X ADD 0920, 092X X ADD 2011 Code 2012 Code Description Car seat/bed testing for airway integrity, neonate, with continual nursing observation and continuous recording of pulse oximetry, heart rate and respiratory rate, with 94780 interpretation and report; 60 minutes Car seat/bed testing for airway integrity, neonate, with continual nursing observation and continuous recording of pulse oximetry, heart rate and respiratory rate, with interpretation and report; each additional full 30 minutes (List separately in addition to code for primary 94781 procedure) Do not report 94780 for less than 60 minutes Will be used primarily for inpatient reporting prior to discharge 218 Payment Rate Comparison October 2011 to 2012 HCPCS Code 94010 94011 94012 94375 94453 94681 Short Descriptor Breathing capacity test Spirometry up to 2 yrs old Spirmtry w/brnchdil inf-2 yr Respiratory flow volume loop Hast w/oxygen titrate Exhaled air analysis o2/co2 SI X X X X X X APC 0367 0367 0367 0367 0367 0369 Relative Weight 0.7420 0.7420 0.7420 0.7420 0.7420 2.6868 2012 Payment Rate $51.95 $51.95 $51.95 $51.95 $51.95 $188.12 N a 2011 t Payment Payment i Rate Diff X 59.63 (7.68) X 59.63 (7.68) X 59.63 (7.68) X 59.63 (7.68) X 59.63 (7.68) X 59.63 128.49 219 Supplies Medical Devices Implantables HCPCS code changes Transmittal 2296 – October 2011 Update of the Hospital Outpatient Prospective Payment System (OPPS) • New Device Pass-Through Categories – It has been over a year since CMS introduced a separately payable medical device • C1749 Endoscope, retrograde imaging/illumination colonoscope device (implantable) –Two new categories as of October 1, 2011: • C1830 – Power bone marrow bx needle • C1840 – Telescopic intraocular lens – One new Code as of January 1, 2012 • C1886 – Catheter extravascular tissue ablation, any modality (insertable) 221 Transmittal 2296 – October 2011 Update of the Hospital Outpatient Prospective Payment System (OPPS) C1830 – Powered bone marrow biopsy needle • http://www.diagnosticpathology.org /content/6/1/23 • Power driver and biopsy needle components of the OnControl powered bone marrow sampling system 222 Transmittal 2296 – October 2011 Update of the Hospital Outpatient Prospective Payment System (OPPS) • Device utilizes a battery-powered drill to insert the bone marrow needle into the iliac bone of adult patient with minimal operator exertion. – Resembles a small hand-held drill and drives a single lumen needle set into the bone cavity. Needle set consists of two parts: an outer cannula, 11 gauge x 4 inches long; and a bevel-tip inner stylet used to penetrate the cortex of the bone. – C1830, Revenue code 0272, 0278 223 Transmittal 2296 – October 2011 Update of the Hospital Outpatient Prospective Payment System (OPPS) • Since the introduction of the Jamshidi needle in 1971 (shown), there has been no substantial advancement in marrow sampling technology 224 Transmittal 2296 – October 2011 Update of the Hospital Outpatient Prospective Payment System (OPPS) • http://abcnews.go.com/Health/Vid eo/videoLogin?id=3476757 • http://www.visoncareinc.net/ • The Implantable Telescope Technology platform incorporates wide-angle micro-optical lenses in a Galilean telescope design. Based on this proprietary technology, VisionCare’s lead product (Implantable Miniature Telescope) along with the cornea, enlarges images in front of the eye approximately 2.2 or 2.7 times their normal size (depending on the model used). 225 Transmittal 2296 – October 2011 Update of the Hospital Outpatient Prospective Payment System (OPPS) • The magnification allows central images to be projected onto healthy perimacular areas of the retina instead of the macula alone, where breakdown of photoreceptors and loss of vision has occurred. This helps reduce the blind spot caused as a result of macular degeneration disease. • C1840, Revenue Code 0278 226 Transmittal 2296 – October 2011 Update of the Hospital Outpatient Prospective Payment System (OPPS) • CPT 66982 Extracapsular cataract removal with insertion of intraocular lens prosthesis (1-stage procedure), manual or mechanical technique (eg, irrigation and aspiration or phacoemulsification), complex, requiring devices or techniques not generally used in routine cataract surgery (eg, iris expansion device, suture support for intraocular lens, or primary posterior capsulorrhexis) or performed on patients in the amblyogenic developmental stage or….. 227 Transmittal 2296 – October 2011 Update of the Hospital Outpatient Prospective Payment System (OPPS) • CPT 66984 Extracapsular cataract removal with insertion of intraocular lens prosthesis (1 stage procedure), manual or mechanical technique (eg, irrigation and aspiration or phacoemulsification) – CPT 66982 and 66984 group to APC 0246 • C1840 has a device offset amount of $221.71 to be deducted from payment facility receives for surgical CPT code 228 Transmittal 2291, FY2012 Inpatient Prospective Payment System • AutoLITT – a minimally invasive MRI-guided laser tipped catheter designed to destroy malignant brain tumors with interstitial thermal energy causing immediate coagulation and necrosis of diseased tissue. – New Technology and add-on payment will be eligible until December 2012. Because the 3-year anniversary date for the AutoLITT will occur after FY2012, CMS will continue to make new technology add-on payment until September 30, 2012 229 AutoLITT 230 Transmittal 2291, FY2012 Inpatient Prospective Payment System – HIM drives facility’s add-on reimbursement with the assignment of: • ICD-9-PCS 17.61, Laser Interstitial Thermal Therapy (LITT) of lesion or tissue of brain under guidance – Add-on Reimbursement Opportunity $5,300 231 Medical Supplies Rev Code SI Ind Action 0272 A NEW 0272 A NEW 0272 E NEW 2011 Code 2012 Code Description Ostomy pouch, drainable, with extended wear barrier attached, with filter, (1 A5056 piece), each Ostomy pouch, drainable, with extended wear barrier attached, with built in A5057 convexity, with filter, (1 piece), each Mechanical wound suction, disposable, includes dressing, all accessories and A9272 components, each 232 Payment Changes for Biological Wound Products HCPCS Short Descriptor Code C9360 C9361 C9362 C9363 C9364 SurgiMend, neonatal NeuroMend nerve wrap Implnt,bon void filler-strip Integra Meshed Bil Wound Mat Porcine implant, Permacol 2012 SI APC Payment Rate K 9360 $11.23 N N K 9363 $20.60 N N 2011 a Payment t Rate .G 11.44 G 239.46 G 51.87 .G 20.99 G 17.44 233 Durable Medical Equipment/Supplies Other Medical/Surgical Supplies DME, Medical Supplies Rev Code 0290 SI 2011 Ind Action Code E DEL 0290 Y NEW 0290 Y NEW 0290 Y NEW E0571 2012 Code Description Aerosol compressor, battery powered, for use with small volume nebulizer No replacement found Manual wheelchair accessory, push E0988 activiated power assist, Each Power wheelchair accessory, Group 34 Non-Sealed Lead ACID battery, E2358 each Power wheelchair accessory, Group 34 Sealed Lead Acid Battery, Each E2359 (e.g. Gel Cell, Absorbed Glassmat) 235 DME, Medical Supplies Rev Code 0274, 0290 0274, 0290 0274, 0290 SI 2011 Ind Action Code A DEL L1500 A DEL L1510 A DEL L1520 2012 Code Description Thoracic-hip-knee-ankle orthotic (THKAO), mobility frame (Newington, Parapodium types) Thoracic-hip-knee-ankle orthotic (THKAO), standing frame, with or without tray and accessories Thoracic-hip-knee-ankle orthotic (THKAO), swivel walker 236 DME, Medical Supplies Rev Code SI 2011 Ind Action Code 0290 Y DEL 0290 Y NEW 2012 Code Description Shoulder-elbow orthotic (SEO), mobile arm support attached to wheelchair, balanced, adjustable, prefabricated, L3964 includes fitting and adjustment Wheelchair accessory, shoulder elbow, mobile arm support attached to E2626 wheelchair, balanced, adjustable 237 DME, Medical Supplies Rev Code SI 2011 Ind Action Code 0290 Y DEL 0290 Y NEW 2012 Code Description Shoulder-elbow orthotic (SEO), mobile arm support attached to wheelchair, balanced, adjustable Rancho type, prefabricated, includes fitting and L3965 adjustment Wheelchair accessory, shoulder elbow, mobile arm support attached to wheelchair, balanced, adjustable E2627 Rancho type 238 DME, Medical Supplies Rev Code SI 2011 Ind Action Code 0290 Y DEL 0290 Y NEW 2012 Code Description L3966 E2628 Shoulder-elbow orthotic (SEO), mobile arm support attached to wheelchair, balanced, reclining, prefabricated, includes fitting and adjustment Wheelchair accessory, shoulder elbow, mobile arm support attached to wheelchair, balanced, reclining 239 DME, Medical Supplies Rev Code SI 2011 Ind Action Code 0290 Y DEL 0290 Y NEW 2012 Code Description L3968 E2629 Shoulder-elbow orthotic (SEO), mobile arm support attached to wheelchair, balanced, friction arm support (friction dampening to proximal and distal joints), prefabricated, includes fitting and adjustment Wheelchair accessory, shoulder elbow, mobile arm support attached to wheelchair, balanced, friction arm support (Friction dampening to proximal and distal joints) 240 DME, Medical Supplies Rev Code SI 2011 Ind Action Code 0290 Y DEL 0290 Y NEW 2012 Code Description Shoulder-elbow orthotic (SEO), mobile arm support, monosuspension arm and hand support, overhead elbow forearm hand sling support, yoke type suspension support, prefabricated, L3969 includes fitting and adjustment Wheelchair accessory, shoulder elbow, mobile arm support, monosuspension arm and hand support, overhead elbow forearm hand E2630 sling support, Yoke type 241 DME, Medical Supplies Rev Code SI 2011 Ind Action Code 0290 Y DEL 0290 Y NEW 2012 Code Description Shoulder-elbow orthotic (SEO), addition to mobile arm support, L3970 elevating proximal arm Wheelchair accessory, addition to mobile arm support, elevating proximal E2631 arm 242 DME, Medical Supplies Rev Code 0290 0290 2011 SI Ind Action Code Y Y DEL NEW L3972 2012 Code Description Shoulder-elbow orthotic (SEO), addition to mobile arm support, offset or lateral rocker arm with elastic balance control Wheelchair accessory, addition to mobile arm support, offset or lateral E2632 rocker arm with elastic balance control 243 DME, Medical Supplies Rev Code SI 2011 Ind Action Code 0290 Y DEL 0290 Y NEW 0274, 0290 A DEL 2012 Code Description Shoulder-elbow orthotic (SEO), addition to mobile arm support, L3974 supinator Wheelchair accessory, addition to E2633 mobile arm support, supinator L4380 Pneumatic knee splint, prefabricated, includes fitting and adjustment 244 DME, Medical Supplies Rev Code 0274, 0290 0274, 0290 SI 2011 Ind Action Code A A DEL NEW L5311 2012 Code Description Knee disarticulation (or through knee), molded socket, external knee joints, shin, SACH foot, endoskeletal system Knee disarticulation (or through knee), molded socket, single axis knee, L5312 Pylon, Sach foot, Endoskeletal System 245 DME, Medical Supplies Rev Code 0274, 0290 0274, 0290 SI 2011 Ind Action Code A A NEW 2012 Code Description Terminal device, multiple articulating digit, includes motor®, initial issue or L6715 replacement NEW Electric hand, switch or myoelectric controlled, independently articulating digits, any grasp pattern or comination L6880 of grasp patterns, includes motor(s) 246 DME, Medical Supplies Rev SI Code Ind Action 0274, 0290 A DEL 0274, 0290 A DEL 0274, 0290 A DEL 0274, 0290 A DEL 2011 Code 2012 Code Description L7266 Servo control, Steeper or equal L7272 Analogue control, UNB or equal Proportional control, 6-12 volt, Liberty, Utah or equal Repair of prosthetic device, hourly rate (excludes V5335 repair of oral or laryngeal prosthesis or artificial larynx) L7274 L7500 247 Neurology Services CPT and HCPCS code changes Sleep Medicine Testings • Provided descriptions for reporting sleep testings: – Actigraphy – Attended – Electrooculogram (EOG) – Maintenance of wakefulness test (MWT) – Multiple sleep latency test (MSLT) – Peripheral arterial tonometry (PAT) – Physiological measurement of sleep – Polysomnography – Sleep Staging 249 Neurology Services Rev Code SI Ind Action 2011 Code 0920, 092X Rev S ADD SI Action 2011 0920, 092X S ADD 2012 Code Description Needle electromyography, each extremity, with related paraspinal areas, when performed, done with nerve conduction, amplitude and latency/velocity study; limited (List separately in 95885 addition to code for primary procedure) 2012 Description Needle electromyography, each extremity, with related paraspinal areas, when performed, done with nerve conduction, amplitude and latency/velocity study; complete, five or more muscles studied, innervated by three or more nerves or four or more spinal levels (List separately in addition to code for primary 95886 procedure) Use 95885, 95886 in conjunction with 9590095904 Do not report 95885, 95886 in conjunction with 95860-95864, 95870, 95905) 250 Neurology Services Rev Code SI Ind 0920, 092X S Action ADD 2011 Code 2012 Code Description Needle electromyography, non-extremity (cranial nerve supplied or axial) muscle(s) done with nerve conduction, amplitude and latency/velocity study (List separately in addition to code for primary 95887 procedure) Use 95887 in conjunction with 95900-95904. Do not report 95887 in conjunction with 95867-95870, 95887 95905. 251 Neurology Services Rev Code SI Ind Action 0920, 0929 S DESC 2011 Code 2012 Code Description Electronic analysis of implanted neurostimulator pulse generator system (eg, rate, pulse amplitude, pulse duration, configuration of wave form, battery status, electrode selectability, output modulation, cycling, impedance and patient compliance measurements); simple or complex brain, spinal cord, or peripheral (ie, cranial nerve, peripheral nerve, sacral nerve, neuromuscular) neurostimulator pulse 95970 generator/transmitter, without reprogramming I 252 Neurology Services Rev Code SI Ind Action 0920, 0929 S DESC 2011 Code 2012 Code Description Electronic analysis of implanted neurostimulator pulse generator system (eg, rate, pulse amplitude, pulse duration, configuration of wave form, battery status, electrode selectability, output modulation, cycling, impedance and patient compliance measurements); simple spinal cord, or peripheral (ie, peripheral nerve, sacral nerve, neuromuscular) neurostimulator pulse generator/transmitter, with intraoperative or 95971 subsequent programming 253 Neurology Services Rev Code SI Ind Action 0920, 0929 S DESC 2011 Code 2012 Code Description Electronic analysis of implanted neurostimulator pulse generator system (eg, rate, pulse amplitude, pulse duration, configuration of wave form, battery status, electrode selectability, output modulation, cycling, impedance and patient compliance measurements); complex spinal cord, or peripheral (ie, peripheral nerve, sacral nerve, neuromuscular) (except cranial nerve) neurostimulator pulse generator/transmitter, with intraoperative or 95972 subsequent programming, first hour 254 Neurology Services Rev Code SI Ind Action 0920, 0929 S DESC 2011 Code 2012 Code Description Electronic analysis of implanted neurostimulator pulse generator system (eg, rate, pulse amplitude, pulse duration, configuration of wave form, battery status, electrode selectability, output modulation, cycling, impedance and patient compliance measurements); complex spinal cord, or peripheral (ie, peripheral nerve, sacral nerve, neuromuscular) (except cranial nerve) neurostimulator pulse generator/transmitter, with intraoperative or subsequent programming, each additional 30 minutes after first hour (List separately in addition 95973 to code for primary procedure) 255 Neurology Services Rev Code SI Ind Action 0920, 0929 S DESC 2011 Code 2012 Code Description Electronic analysis of implanted neurostimulator pulse generator system (eg, rate, pulse amplitude, pulse duration, configuration of wave form, battery status, electrode selectability, output modulation, cycling, impedance and patient compliance measurements); complex cranial nerve neurostimulator pulse generator/transmitter, with intraoperative or subsequent programming, with or without nerve 95974 interface testing, first hour 256 Neurology Services Rev Code SI Ind Action 0920, 0929 S DESC 2011 Code 2012 Code Description Electronic analysis of implanted neurostimulator pulse generator system (eg, rate, pulse amplitude, pulse duration, configuration of wave form, battery status, electrode selectability, output modulation, cycling, impedance and patient compliance measurements); complex cranial nerve neurostimulator pulse generator/transmitter, with intraoperative or subsequent programming, each additional 30 minutes after first hour (List separately in addition 95975 to code for primary procedure) 257 Neurology Services Rev Code SI Ind 0922, 0929 S 0922, 0929 S ADD 2012 Code Description Short-latency somatosensory evoked potential study, stimulation of any/all peripheral nerves or skin sites, recording from the central nervous 95938 system; in upper and lower limbs ADD Central motor evoked potential study (transcranial 95939 motor stimulation); upper and lower limbs Action 2011 Code 258 Neurology Services Rev Code SI Ind 0920, 0929 S 0920, 0929 S ADD 2012 Code Description Percutanous or open implantation of neurostimulator electrode array(s), subcutaneous (peripheral subcutaneous field stimulation), including imaging guidance, when performed, cervical, thoracic or lumbar; for trial, including 0282T removal at the conclusion of trial period ADD Percutanous or open implantation of neurostimulator electrode array(s), subcutaneous (peripheral subcutaneous field stimulation), including imaging guidance, when performed, cervical, thoracic or lumbar; permanent, with 0283T implantation of a pulse generator Action 2011 Code Included in Interventional Radiology Section, discussed earlier 259 Neurology Services Rev Code SI Ind 0920, 0929 T ADD 0920, 0929 S ADD Action 2011 Code 2012 Code Description Revision or removal of pulse generator or electrodes, including imaging guidance, when performed, including addition of new electrodes, 0284T when performed Electronic analysis of implanted peripheral subcutaneous field stimulation pulse generator, 0285T with reprogramming when performed 260 Clinic/Outpatient Departments CPT and HCPCS code changes Clinic/Outpatient Departments • Revised paragraphs in section entitled: –“Immunization Administration for Vaccines/Toxoids” • In 2011 states “patient and family” • In 2012 states “patient/family” in all locations –Clarification provided for multi-valent antigens and conjugates or adjuvants contained in vaccines 262 Clinic/Outpatient Departments Rev Code SI 2011 Ind Action Code 0771 B 0771 B 2012 Code Description Immunization administration through 18 years of age via any route of administration, with counseling by physician or other qualified health care professional; first vaccine/toxoid DESC 90460 component Immunization administration through 18 years of age via any route of administration, with counseling by physician or other qualified health care professional; first or only component DESC 90460 of each vaccine or toxoid administered 263 Clinic/Outpatient Departments Rev Code 0771 0771 Rev 0771 2012 Code Description Immunization administration through 18 years of age via any route of administration, with counseling by physician or other qualified health care professional; each additional vaccine/toxoid component (List separately in addition to code for primary procedure) B DESC 90461 Immunization administration through 18 years of age via any route of administration, with counseling by physician or other qualified health care professional; each additional vaccine or toxoid administered (List separately in addition to code for procedure) B DESC Description 2012 primary Action 2011 90461 SI H1N1 immunization administration (intramuscular, intranasal), including counseling when performed 90470 DEL 2011 SI Ind Action Code CPT 90470 listed as deleted in Appendix B Transmittal #2174, effective 4/1/11 listed both CPT 90470 and 90663 as being deleted, effective 1/1/2011 264 Clinic/Outpatient Departments Rev Code 051X, 0761, 094X 051X, 0761, 094X 051X, 0761, 094X SI 2011 Ind Action Code N DEL 92070 2012 Code Description Fitting of contact lens for treatment of disease, including supply of lens N NEW Fitting of contact lens for treatment of occular surface 92071 disease N NEW Fitting of contact lens for management of keratoconus, initial 92072 fitting For subsequent fittings, report using Evaluation and Management services or General Ophthalmological services 265 Clinic/Outpatient Departments Rev Code 051X, 0761, 092X 051X, 0761, 092X SI 2011 Ind Action Code 2012 Code Description S DEL 92120 Tonography with interpretation and report, recording indentation tonometer method or perilimbal suction method S DEL 92130 Tonography with water provocation Single episode tonometry is a component of general ophthalmological Services, reported with E/M services, e.g.: 92002-92004,. 92012-92014, 92100, 99211-99480 266 Clinic/Outpatient Departments Rev Code 0335, 094X 0335, 094X SI 2011 2012 Ind Action Code Code Description Refilling and maintenance of implantable pump or reservoir for drug delivery, spinal (intrathecal, epidural) or brain S DESC 95990 (intraventricular); Refilling and maintenance of implantable pump or reservoir for drug delivery, spinal (intrathecal, epidural) or brain (intraventricular), includes electronic analysis of pump, S DESC 95990 when performed; 267 Clinic/Outpatient Departments Rev Code 0335, 094X 0335, 094X SI 2011 2012 Ind Action Code Code Description Refilling and maintenance of implantable pump or reservoir for drug delivery, spinal (intrathecal, epidural) or brain S DESC 95991 (intraventricular); administered by physician Refilling and maintenance of implantable pump or reservoir for drug delivery, spinal (intrathecal, epidural) or brain (intraventricular), includes electronic analysis of pump, S DESC 95991 when performed; requiring physician's skill 268 Clinic/Outpatient Departments Rev SI 2011 2012 Code Ind Action Code Code Description 036X, 051X, Rhinophototherapy, intranasal application of ultraviolet and 0761 T DEL 0168T visible light, bilateral Replacement CPT code 30999 Rhinophototherapy for the treatment of allergies is not medically necessary due to insufficient clinical evidence in the peer-reviewed medical literature. Medicare does not have a National Coverage Determination or a Local Coverage Determination for Rhinophototherapy. 269 Clinic/Outpatient Departments Rev Code 051X, 0761 051X, 0761 051X, 0761 051X, 0761 051X, 0761 SI 2011 Ind Action Code E DEL 11975 DEL Insertion, implantable contraceptive capsules 11981 Insertion, non-biodegradable drug delivery implant X Exist E 2012 Code Description 11977 Removal with reinsertion, implantable contraceptive capsules X Exist 11981 Insertion, non-biodegradable drug delivery implant T Exist 11976 Removal, implantable contraceptive capsules 270 Clinic/Outpatient Departments Rev Code 051X, 076X 051X, 076X SI 2011 Ind Action Code 2012 Code Description Electronic analysis of programmable, implanted pump for intrathecal or epidural drug infusion (includes evaluation of reservoir status, alarm status, drug S DESC 62367 prescription status); without reprogramming Electronic analysis of programmable, implanted pump for intrathecal or epidural drug infusion (includes evaluation of reservoir status, alarm status, drug S DESC 62367 prescription status); without reprogramming or refill 271 Clinic/Outpatient Departments Rev Code 051X, 076X 051X, 076X SI 2011 Ind Action Code S S 2012 Code Description ADD Electronic analysis of programmable, implanted pump for intrathecal or epidural drug infusion (includes evaluation of reservoir status, alarm status, drug 62369 prescription status); with reprogramming and refill ADD Electronic analysis of programmable, implanted pump for intrathecal or epidural drug infusion (includes evaluation of reservoir status, alarm status, drug prescription status); with reprogramming and refill 62370 (requiring physician's skill) 272 Clinic/Outpatient Departments Rev Code 051X, 076X SI Ind S Action ADD 2011 Code 2012 Code Description Transcutaneous electrical modulation pain reprocessing (eg, scrambler therapy), each treatment session (includes placement of 0278T electrodes) A type of treatment for nerve pain that uses electrodes placed on the skin. Electricity is carried from the electrodes through the skin and blocks the pain. The pain may be caused by physical injury, infection, toxic substances, and certain diseases or drugs, including anticancer drugs. 273 Clinic/Outpatient Departments Rev SI 2011 Cod Ind Action Code 0949 A DEL G9041 0949 A DEL G9042 0949 A DEL G9043 0949 A DEL G9044 2012 Code Description Rehabilitation services for low vision by qualified occupational therapist, direct one-on-one contact, each 15 minutes Rehabilitation services for low vision by certified orientation and mobility specialists, direct one-on-one contact, each 15 minutes Rehabilitation services for low vision by certified low vision rehabilitation therapist, direct one-on-one contact, each 15 minutes Rehabilitation services for low vision by certified low vision rehabilitation teacher, direct one-on-one contact, each 15 minutes 274 GI Laboratory CPT and HCPCS code changes GI Laboratory Rev SI 2011 2012 Code Ind Action Code Code Description 0750, 092X 0750, 092X Esophageal motility (manometric study of the esophagus and/or gastroesophageal junction) study X DESC 91010 with interpretation and report; 2-dimensional data Esophageal motility (manometric study of the esophagus and/or gastroesophageal junction) study X DESC 91010 with interpretation and report 276 GI Laboratory Rev SI 2011 2012 Code Ind Action Code Code Description Esophageal motility (manometric study of the 0750, esophagus and/or gastroesophageal junction) study; 092X X DEL 91012 with acid perfusion studies Code Listed as “Deleted” in 2012 Code actually deleted January 2011 Replacement codes see 91013 with 91010 277 GI Laboratory Rev SI 2011 2012 Code Ind Action Code Code Description Esophageal motility (manometric study of the esophagus and/or gastroesophageal junction) study with interpretation and report; with stimulation or 051X, perfusion during 2-dimensional data study (eg, 0750, stimulant, acid or alkali perfusion) (List separately in 092X X DESC 91013 addition to code for primary procedure) Esophageal motility (manometric study of the esophagus and/or gastroesophageal junction) study 051X, with interpretation and report; with stimulation or 0750, perfusion(eg, stimulant, acid or alkali perfusion) (List 092X X DESC 91013 separately in addition to code for primary procedure) 278 GI Laboratory Rev SI 2011 2012 Code Ind Action Code Code Description 0750, 092X 0750, 092X Esophageal motility (manometric study of the esophagus and/or gastroesophageal junction) study with interpretation and report; with 3-dimensional X DESC 0240T high resolution esophageal pressure topography Esophageal motility (manometric study of the esophagus and/or gastroesophageal junction) study with interpretation and report; with high resolution X DESC 0240T esophageal pressure topography 279 GI Laboratory Rev SI 2011 2012 Code Ind Action Code Code Description 0750, 092X 0750, 092X Esophageal motility (manometric study of the esophagus and/or gastroesophageal junction) study with interpretation and report; with stimulation or perfusion during 3-dimensional high resolution esophageal pressure topography study (eg, stimulant, acid or alkali perfusion) (List separately in X DESC 0241T addition to code for primary procedure) Esophageal motility (manometric study of the esophagus and/or gastroesophageal junction) study with interpretation and report; with stimulation or perfusion during high resolution esophageal pressure topography study (eg, stimulant, acid or alkali perfusion) (List separately in addition to code for X DESC 0241T primary procedure) 280 GI Laboratory Rev SI 2011 2012 Code Ind Action Code Code Description Bronchoscopic bronchial thermoplasty with image 0750, guidance (if performed), radiofrequency ablation of 092X T DEL C9730 airway smooth muscle, 1 lobe Bronchoscopy, rigid or flexible, including fluoroscopic 0750, guidance, when performed; with bronchial 092X T NEW Rev SI Action 2011 0276T 2012 thermoplasty, Description 1 lobe 0750, 092X 0750, 092X Bronchoscopic bronchial thermoplasty with image C9731 guidance (if performed), radiofrequency ablation of T DEL ` airway smooth muscle, 2 or more lobes Bronchoscopy, rigid or flexible, including fluoroscopic guidance, when performed; with bronchial T NEW 0277T thermoplasty, 2 or more lobes http://www.btforasthma.com/video/procedure.htm 281 GI Laboratory Rev SI 2011 2012 Code Ind Action Code Code Description 0750, 092X T ADD Anoscopy, with delivery of thermal energy to the 0288T muscle of the anal canal (eg, for fecal incontinence) 282 Rehabilitation Services: Audiology, Physical Speech and Occuptational Therapy CPT and HCPCS code changes Rehabilitation Services – Audiology Rev Code 0471 SI Ind Action E ADD 2011 Code 2012 Code Description Evoked otoacoustic emissions, screening (qualitative measurement of distortion product or transient evoked otoacoustic emissions), 92558 automated analysis THIS MAY BE THE NEW "NEWBORN HEARING SCREENING" TEST CODE, MOST HAVE USED THE CURRENT 92585 284 Rehabilitation Services - Audiology Rev Code SI Ind Action 0471 X DESC 0471 X DESC 2011 Code 2012 Code Description Evoked otoacoustic emissions; limited (single stimulus level, either transient or distortion 92587 products) Distortion product evoked otoacoustic emissions; limited evaluation to confirm the presence or absence of hearing disorder, 3-6 frequencies) or transient evoked otoacoustic 92587 emissions, with interpretation and report 285 Rehabilitation Services - Audiology Rev Code SI Ind Action 0471 X DESC 0471 X DESC 2011 Code 2012 Code Description Evoked otoacoustic emissions; comprehensive or diagnostic evaluation (comparison of transient and/or distortion product otoacoustic emissions at multiple levels and 92588 frequencies) Distortion product evoked otoacoustic emissions; comprehensive or diagnostic evaluation (quantitative analysis of outer hair cell function by cochlear mapping, minimum of 12 frequencies), with 92588 interpretation and report 286 Rehabilitation Services – Audiology Rev Code SI Ind Action 0471 N DESC 0471 N DESC 2011 Code 2012 Code Description Evaluation of central auditory function, with 92621 report; each additional 15 minutes Evaluation of central auditory function, with report; each additional 15 minutes (List separately in addition to code for primary 92621 procedure) 287 Rehabilitation Services Rev Code 051X, 076X, 0420, 0430 051X, 076X, 0420, 0430 SI 2011 2012 Ind Action Code Code Description X NEW X NEW Extracorporeal shock wave for integumentary wound healing, high energy, including topical 0299T application and dressing care; initial wound Extracorporeal shock wave for integumentary wound healing, high energy, including topical application and dressing care; each additional wound (List separately in addition to code for 0300T primary procedure) Effective January 1, 2012, not printed in new code book. See also 0019T, 0101T and 0102T Discussed previously in Wound Clinic section 288 Rehabilitation Services – Speech Therapy Rev Code SI Ind Action 0444 A DESC 0444 A DESC 2011 Code 2012 Code Description Evaluation for prescription of non-speechgenerating augmentative and alternative 92605 communication device Evaluation for prescription of non-speechgenerating augmentative and alternative communication device, face-to-face with the 92605 patient; first hour 289 Rehabilitation Services – Speech Therapy Rev Code 0444 SI Ind Action A ADD 2011 Code 2012 Code Description Evaluation for prescription of non-speechgenerating augmentative and alternative communication device, face-to-face with the patient; each additional 30 minutes (List separately in addition to code for primary 92618 procedure) 290 Rehabilitation Services-Physical/Occupational Therapy Rev Code 042X, 043X, 042X, 043X, SI Ind Action S DESC S DESC 2011 Code 2012 Code Description Application of multi-layer venous wound 29581 compression system, below knee Application of multi-layer compression system; 29581 leg (below knee), including ankle and foot 291 Rehabilitation Services-Physical/Occupational Therapy Rev Code 042X, 043X, 042X, 043X, 042X, 043X, SI Ind Action S ADD S ADD S ADD 2011 Code 2012 Code Description Application of multi-layer compression system; thigh and leg, including ankle and foot, when 29582 performed Application of multi-layer compression system; 29583 upper arm and forearm Application of multi-layer compression system; 29584 upper arm, forearm, hand, and fingers 292 Miscellaneous Codes CPT and HCPCS code changes Nursery, Nursing Procedures Rev SI Code Ind Action 0761 N ADD 0761 N ADD 2011 Code 2012 Code Description Total body systemic hypothermia, per day, in the 0260T neonate 28 days of age or younger Selective head hypothermia, per day, in the neonate 28 0261T days or younger Codes were introduced January, 2011. Printed code in new code book, listed as new code for 2012. 294 Nursing Procedures Rev SI 2011 2012 Code Ind Action Code Code Description Near-infrared guidance for vascular access requiring real-time digital visualization of subcutaneous vasculature for evaluation of potential access sites and 092X N ADD 0287T vessel patency 295 Nursing Procedures 296 Nursing Procedures Rev SI Code Ind Action 0920, 092X X ADD 0920, 092X X ADD 2011 Code 2012 Code Description Car seat/bed testing for airway integrity, neonate, with continual nursing observation and continuous recording of pulse oximetry, heart rate and respiratory rate, with 94780 interpretation and report; 60 minutes Car seat/bed testing for airway integrity, neonate, with continual nursing observation and continuous recording of pulse oximetry, heart rate and respiratory rate, with interpretation and report; each additional full 30 minutes (List separately in addition to code for primary 94781 procedure) 297 Surgery Procedures CPT and HCPCS code changes Skin Substitute Applications • Classification of repairs: –Simple repair –Intermediate repair –Complex repair • When more than one classification of wounds is repaired, list the more complicated as the primary procedure and the less complicated as the secondary procedure, using modifier 59 299 In-Patient Only Procedure Revisions, 2012 HCPCS Short Descriptor Code G0406 G0407 G0408 G0425 G0426 G0427 Inpt/tele follow up 15 Inpt/tele follow up 25 Inpt/tele follow up 35 Inpt/ED teleconsult30 Inpt/ED teleconsult50 Inpt/ED teleconsult70 Relative Payment SI APC Weight Rate B B B B B B Inpatient Only for 2011 G C G C G C G C G C G C 300 In-Patient Only Procedure Revisions, 2012 HCPCS Short Descriptor Code 20930 20931 21346 22551 22554 35045 43281 43770 54650 0184T Sp bone algrft morsel add-on Sp bone algrft struct add-on Treat nose/jaw fracture Neck spine fuse&remov bel c2 Neck spine fusion Repair defect of arm artery Lap paraesophag hern repair Lap place gastr adj device Orchiopexy (Fowler-Stephens) Exc rectal tumor endoscopic Inpatient Only for 2011 C C $1,742.66 C $3,552.21 C $3,552.21 C $2,181.03 C $5,010.75 C $3,356.53 C $2,305.58 C 0 $1,711.07 C 1 Relative Payment SI APC Weight Rate N N T T T T T T T T 254 208 208 93 132 131 154 149 24.89 50.734 50.734 31.151 71.566 47.939 32.929 24.438 301 New Procedures for 2012 Introduced as SI of “C” HCPCS Code 22633 22634 32096 32097 32098 32505 32506 32507 32666 32667 Short Descriptor SI Lumbar spine fusion combined Spine fusion extra segment Open wedge/bx lung infiltr Open wedge/bx lung nodule Open biopsy of lung pleura Wedge resect of lung initial Wedge resect of lung add-on Wedge resect of lung diag Thoracoscopy w/wedge resect Thoracoscopy w/w resect addl C C C C C C C C C C HCPCS Code 32668 32669 32670 32671 32672 32673 32674 0281T 0293T 0294T Short Descriptor SI Thoracoscopy w/w resect diag Thoracoscopy remove segment Thoracoscopy bilobectomy Thoracoscopy pneumonectomy Thoracoscopy for lvrs Thoracoscopy w/thymus resect Thoracoscopy lymph node exc Laa closure w/implant Ins lt atrl press monitor Ins lt atrl press mont addon C C C C C C C C C C 302 Chargemaster Revision Process • Utilize Current CDM – Review all procedures – Revise as needed: • Additions • Deletions • HCPCS code changes • Revenue code changes • Descriptor revisions – Update encounter forms/input documents – Data transfer issues 303 Coding Decisions • Share Chargemaster with coding staff – Eliminates duplicate work – Avoids potential double reporting – Assures continuity of reporting procedures and proper revenue 304 Final Thoughts • Embrace the future…… – Look forward to updating the Chargemaster • New challenges • New codes • New reimbursement opportunities??? • No one is doing “everything” correctly 305 Final Thoughts • Read parenthetical statements – Green font • Refer to “Coding Tips” – Read all paragraphs preceding sections • Update Charge tickets or orderable screens • Review productivity measures • Educate • Continually monitor gross revenue charges – Monitor Revenue Capture processes for vulnerable clinical areas 306 Final Thoughts • Mastering change is key element for success • 2012 offers new challenges –Good luck!!! 307 Biography • Glenda J. Schuler, RHIT, CPC, CPC-H – glenda.schuler@optum.com – Senior chargemaster consultant for Ingenix – Over 30 years of experience in billing, coding, chargemaster, CPT coding – Past National speaker for AAPC, AHIMA, state hospital associations, state HIMA chapters, VHA, HFMA, Ingenix and other organizations specific for: – Ambulatory payment classifications – Chargemasters – OCE Editor and CCI reporting – Modifiers 308