ch5_challenge
advertisement

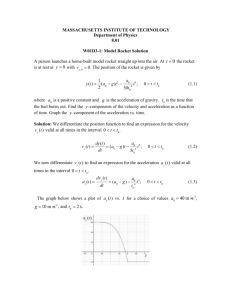
Chapter 5 – Rocket Challenge. Your Challenge is to build a bottle rocket that will go as high as possible. You have 3 choices as to how to launch your rocket. You will be graded on style, the height obtained and a detailed explanation of the chemistry involved. All groups must use a 2 L soda bottle, and all rockets will be launched using the same launchers. Choices: 1. Air Pressure rocket. The rocket is pumped up with air and released. You need to include calculations of pressure and explain how your rocket is propelled upwards. 2. Water pressure rocket. The rocket is filled about ½ way with water and the pressure is increased. You need to investigate the best proportions of water to air and give a detailed explanation of how the rocket is propelled upwards. 3. Hydrogen- Oxygen rocket. The rocket is filled with a mixture of hydrogen and oxygen and ignited with a spark. You need to include the chemistry of the reaction (including how H2 and O2 are formed, and the ratios used) and how this is used to propel the rocket upwards. Air Pressure Rocket. 1. 2. 3. 4. Calculate the volume contained in 1 pump of the bicycle pump. Measure the exact volume of the bottle. Count the # of pumps required to launch the rocket. Calculate the pressure of the gas inside the bottle using the volume of air pumped into it and Boyles Law (P1V1=P2V2) 5. Using the ideal gas law, how many moles of air are in your bottle? 6. Assuming air is 80% nitrogen and 20% oxygen, how many moles of each are in your bottle? 7. Measure the mass of the bottle before being pumped full of air. 8. Using the molar mass of nitrogen and oxygen, calculate the mass of the bottle after it is pumped full of air. 9. Calculate the pressure of your rocket at O ˚C and 50 ˚C. 10. What volume would the gas take up at these temperatures? 11.Explain what happens, in terms of pressure and volumes of the air when the launch trigger is pulled. 12.Would you expect your rocket to go higher or lower at 50 ˚C than at O˚C? Explain your reasoning. Data Temp: ___________________ Pressure ______________________ Volume of rocket _______________________________________ Volume of 1 pump (air)___________________________________ Mass of empty rocket____________________________________ # of pumps needed to launch_______________________________ Height rocket reached ___________________________________ Water Pressure Rocket. 1. Measure the volume contained in 1 pump of the bicycle pump. 2. Research on the internet, the effect of different volumes of water in your rocket. Choose an amount of water you want to put in your bottle. 3. If this much water is in the bottle, how much air is in the bottle? 4. Count the # of pumps required to launch the rocket. 5. Calculate the pressure of the gas inside the bottle using the volume of air pumped into it and Boyles Law (P1V1=P2V2) 6. Using the ideal gas law, how many moles of air are in your bottle when it is launched? 7. Assuming air is 80% nitrogen and 20% oxygen, how many moles of each are in your bottle? 8. Using the density of water (1g/ml), and the mass of the empty bottle calculate the total mass of the bottle (including water) before it is pumped full of air. 9. Using the molar mass of nitrogen and oxygen, calculate the mass of the bottle after it is pumped full of air. 10. Explain, in terms of pressure and volume of air and water, what happens when the rocket is launched. 11. How does the addition of water to the rocket affect how far it goes? Compare your results to those of the air rockets and other water rockets. 12. How would the launch be effected by temperature. Would you expect your rocket to go higher or lower on a hot day (40 ˚C) than a cold day ( 0 ˚C) Temp: ___________________ Pressure _____________________ Volume of rocket _______________________________________ Volume of 1 pump (air)___________________________________ Mass of empty rocket____________________________________ Volume of water used____________________________________ # of pumps needed to launch_______________________________ Height rocket reached ____________________________________ Hydrogen- Oxygen Rocket 1. 2. 3. 4. Write a balanced equation for how you produced the hydrogen gas. Write a balanced equation for you produced the oxygen gas. Write a balanced equation for the reaction inside your bottle. What ratio of hydrogen to oxygen did you use to fill your bottle? Why did you choose this ratio? 5. Using the volume of hydrogen you put in the bottle, how many moles of water were produced? 6. Using the volume of oxygen you put in the bottle, how many grams of water were produced? 7. What state was the water you produced (s, l, g?) Where did it go when your rocket was launched? 8. Explain, in terms of energy of the reaction, why your rocket was propelled upwards during the reaction? 9. Is this reaction exothermic or endothermic? Explain how you know. 10. Using the volume of hydrogen you produced and atmospheric pressure and room temperature, calculate the volume the hydrogen would be at 1 atm and 273K. 11.What amount (in L) of hydrogen would be required to produce 2L of water vapor. 12.How many moles of oxygen would be required to fill a 2L bottle to 3 atm of pressure at 35 ˚C? Data Temperature ___________________________ Pressure ____________ Volume of bottle ____________________________________________ Volume of hydrogen used ____________________________________ Volume of Oxygen used ______________________________________ Height reached by rocket ______________________________________