File
advertisement

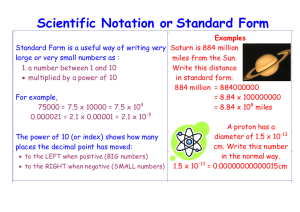
Unit 2 Measurements Matter - anything that occupies space and has mass mass – measure of the quantity of matter SI unit of mass is the kilogram (kg) 1 kg = 1000 g = 1 x 103 g weight – force that gravity exerts on an object weight = mass g (F = ma) on earth, g = 1.0 on moon, g ~ 0.1 La Grande K 1 Kg Pt/Ir alloy World’s Roundest Object https://www.youtube.com/watch?v=ZMByI4s-D-Y A 1 kg bar will weigh 1 kg on earth 0.1 kg on moon International System of Units (SI) Base Units Used in this class and should have memorized All other units are derived from these units and are known as Derived Units Velocity: m/s Force: 1 Newton = 1 kg•m/s2 Volume: m3 Prefixes can be used to simplify for extremely large or small quantities of base units “mu” Used most often in this class, be sure to memorize. Prefix examples Driving 321,000 meters to LR = 321 kilometers Radio station 90.9 MHz = 90,900,000 Hz A mosquito weighs 2.5 milligrams (mg) = 0.0025 grams (g) A dust mite’s length 0.0002 meters = 200 micrometers (mm) Conversion factors can be written/used 2 ways -6 6 10 ML = 1 L 1 Mm = 10 L -3 10 3 10 1km = L Liter (base) -2 1 cL = 10 L -3 1 mL = 10 L -6 1 mL = 10 L -9 1 nL = 10 L Or kL = 1 L Liter (base) 2 10 cL = 1 L 3 10 mL = 1 L 6 10 mL = 1 L 9 10 nL = 1 L I favor the forms using (+) exponents Volume – SI derived unit for volume is cubic meter (m3) (cm3 is commonly used) 1 cm3 = 1 mL Density – SI derived unit for density is kg/m3 1 g/cm3 = 1 g/mL = 1000 kg/m3 *more commonly used density = mass volume m d= V 2 L of Os = 100 lbs Example 1.1 Gold is a precious metal that is chemically unreactive. It is used mainly in jewelry, dentistry, and electronic devices. A piece of gold ingot with a mass of 301 g has a volume of 15.6 cm3. Calculate the density of gold. gold ingots Example 1.2 The density of mercury, the only metal that is a liquid at room temperature, is 13.6 g/mL. Calculate the mass of 5.50 mL of the liquid. m d= V Chemistry In Action On 9/23/99, $125M Mars Climate Orbiter entered Mars’ atmosphere 100 km (62 miles) lower than planned and was destroyed by heat. 1 lb = 1 N 1 lb = 4.45 N Failed to convert English to metric units “This is going to be the cautionary tale that will be embedded into introduction to the metric system in elementary school, high school, and college science courses till the end of time.” Scientific Notation The number of atoms in 12 g of carbon: 602,200,000,000,000,000,000,000 6.022 x 1023 The mass of a single carbon atom in grams: 0.0000000000000000000000199 1.99 x 10-23 We can factor out powers of 10 to simplify very large or small numbers N is the base number between 1 and 10 N x 10n Exponent (n) is a positive or negative integer Scientific Notation • Base x 10exponent • Base number ≥ 1 and < 10 5 320,000 = 3.2 x 10 . 0.000074 . = 7.4 x 10-5 Decimal moved left so (+) Decimal moved right so (–) Scientific Notation Practice Write these in scientific notation • 0.00578 • 579 • 96,000 • 0.0140 Write these in long notation • 2.0 x 103 • 3.58 x 10-4 • 4.651 x 107 • 9.87 x 10-2 Mathematics in Scientific Notation Addition or Subtraction: Must have same exponent 1. Write each quantity with the same exponent n 2. Combine N1 and N2 4.31 x 104 + 3.9 x 103 = 4.31 x 104 + 0.39 x 104 = 3. The exponent, n, remains the same 4.70 x 104 Tip: change smaller number to match larger exponent 1.36 x 10-1 – 4 x 10-3 = 1.36 x 10-1 – 0.04 x 10-1 = = 1.32 x 10-1 More practice: 1.45 x 10601 + 2.4 x 10600 Mathematics in Scientific Notation Multiplication: Add exponents 1. Multiply N1 and N2 2. Add exponents n1 and n2 Practice: (3 x 10250) (7.2 x 10200 ) (4.0 x 10-5) x (7.0 x 103) = (4.0 x 7.0) x (10-5+3) = 28 x 10-2 = 2.8 x 10-1 Division: Subtract exponents 1. Divide N1 and N2 2. Subtract exponents n1 and n2 Practice: 9.5 x 1045) ÷ (3.7 x 1050) 8.5 x 104 ÷ 5.0 x 109 = (8.5 ÷ 5.0) x 104-9 = 1.7 x 10-5 Bell Ringer a) Write in scientific notation • 8,705,000 m • 0.0000045 L • 0.00237 sec • 9,300 g b) Rewrite above numbers using the nearest SI prefix c) Perform the below mathematics in Sci. Notation • (9.01 x 103 g) + (3.8 x 102 g) • (2.61 x 107 m) x (9.87 x 10-2 m) • (3.98 x 10-2 m) – (8.2 x 10-3 m) • (8.4 x 109 g) ÷ (2.0 x 104 mL) Precision indicates to what degree we know our measurement. (Arithmetic precision) A measurement of 18.0 grams could be made on an average countertop food scale (balance). (~$20) A high-precision milligram scale could weigh the same sample with a much higher precision (18.0235 grams) (~$1,500) Every measurement is limited by the equipment’s level of precision. (Never exact) e.g. mass of hydrogen atom: 0.0000000000000000000000017 grams Significant Figures: Used to prevent uncertainty from rounding of various measured quantities with various levels of precision. 1) Any digit that is not zero is significant 1.234 kg 34,000 mm 4 significant figures 2 significant figures 2) Zeros between nonzero digits are significant 606 cm 50,050 s 3 significant figures 4 significant figures 3) Zeros to the left of the first nonzero digit are not significant 0.08 mL 0.00054 ML 1 significant figure 2 significant figures 4) If a number is greater than 1, then all zeros to the right of the decimal point are significant 2.0 mg 20.000 g 2 significant figures 5 significant figures 5) If a number is less than 1, then only the zeros at the end are significant 0.00420 g 3 significant figures 0.1000 g 4 significant figures Significant Figures Every significant figure is shown when using Scientific notation. ____ m 0.001400 4 significant figures 1.400 x -3 10 Not 1.4 x -3 10 __ mL 500 2 significant figures 5.0 x 2 10 Not 5 x 2 10 Exampl 1.4 Unit Conversions e Determine the number of significant figures in the following measurements: (a) 478 cm (d) 0.0430 kg 22 10 (b) 600,001 g (e) 1.310 × (c) 0.85 m (f) 7000 mL atoms Example 1.4 Solution (a) 478 cm -- Three, because each digit is a nonzero digit. (b) 600,001- Six, because zeros between nonzero digits are significant. (c) 0.825 m -- Three, because zeros to the left of the first nonzero digit do not count as significant figures. (d) 0.0430 kg -- Three. The zero after the nonzero is significant because the number is less than 1. (e) 1.310 × 1022 atoms -- Four, because the number is greater than one so all the zeros written to the right of the decimal point count as significant figures. Example 1.4 solution (f)7000 mL -- This is an ambiguous case. The number of significant figures may be four (7.000 × 103), three (7.00 × 103), two (7.0 × 103), or one (7 × 103). This example illustrates why scientific notation must be used to show the proper number of significant figures. If no decimal is present it is usually assumed only non-zeros are significant. If a decimal is present, than all zero’s are significant. 7,000 mL ≠ 7,000. mL They display differing degrees of precision. Significant Figures Addition or Subtraction The answer cannot have more digits to the right of the decimal point than any of the original numbers. Use the least precise number. 89.392 L + 1.1XX 90.492 ± 50 mL one significant figure after decimal point round off to 90.5 ± 1.0 mL 3.70XX -2.9133 0.7867 two significant figures after decimal point round off to 0.79 Significant Figures Multiplication or Division The number of significant figures in the result is set by the original number that has the smallest number of significant figures. 4.51 x 3.0006 = 13.532706 = 13.5 round to 3 sig figs 3 sig figs 6.8 ÷ 112.04 = 0.0606926 = 0.061 2 sig figs round to 2 sig figs Example 1.5 Carry out the following arithmetic operations to the correct number of significant figures: (a) 11,254.1 g + 0.1983 g (b) 66.59 L − 3.113 L (c) 8.16 m × 5.1355 kg (d) 0.0154 kg ÷ 88.3 mL (e) (2.64 × 103 cm) + (3.27 × 102 cm) Example 1.5 Solution Solution In addition and subtraction, the number of decimal places in the answer is determined by the number having the lowest number of decimal places. (a) (b) Example 1.5 Solution In multiplication and division, the significant number of the answer is determined by the number having the smallest number of significant figures. (c) (d) (e) First we change 3.27 × 102 cm to 0.327 × 103 cm and then carry out the addition (2.64 cm + 0.327 cm) × 103. Following the procedure in (a), we find the answer is 2.97 × 103 cm. Bell Ringer a) Perform the below mathematics in Sci. Notation. using Significant Figures in your answer. 1. (9.8 x 105 g) + (6.75 x 104 g) 2. (5.98 x 10-6 m) – (7 x 10-8 m) b) Rewrite the first 2 solutions using the nearest SI prefix 3. (2.612 x 1010 m) x (9.87 x 10-3 m) 4. (7 x 102 mg) ÷ (1.875 x 104 mL) Significant Figures Exact Numbers Numbers from definitions or numbers of objects are considered to have an infinite number of significant figures. •The average of three measured lengths: 6.64, 6.68 and 6.70? 6.64 + 6.68 + 6.70 3 = 6.67333 = 7 = 6.673 Because 3 is an exact number, not a measured number; It is not used for sigfigs. • How many feet are in 6.82 yards? 6.82 yards x 3 ft/yard = 20.5 ft = 20 ft 1 yard = exactly 3 ft by definition Accuracy – how close a measurement is to the true value Precision – how close a set of measurements are to each other accurate & precise precise but not accurate not accurate & not precise Percent Error A way to determine how accurate your measurements are to a known value. |Obtained value – Actual value| x 100% Actual Value Ranges between 0 and 100% Ex. I weigh a 3 kg block on three different scales: 3.2 kg, 3.0 kg, 3.1 kg = 3.1 kg average 3.1 – 3.0 x 100% = 3.3% error 3.0 Dimensional Analysis of Solving Problems (Train-Tracks) 1. Determine which unit conversion factors are needed 2. Carry units through calculation 3. If all units cancel except for the desired unit(s), then the problem was solved correctly. given quantity x conversion factor = desired quantity given unit x desired unit given unit = desired unit Train Track Example How many inches are in 3.0 miles? Identify beginning information Draw a train track 3 miles Write measurement as a fraction Train Track Example How many inches are in 3.0 miles? • We are going from a larger measurement to a smaller one. • Find a conversion factor you know that changes miles into something smaller. Conversion Factor: 1 mile = 5,280 feet • Write your conversion factor on the track so that miles cancels out and you are left with the unit feet. 3 miles 5280 feet 1 mile Always need same units on opposite sides to cancel out Train Track Example How many inches are in 3.0 miles? We now need another conversion factor between Feet and Inches: 1 foot = 12 inches Again, place conversion factor so that the previous unit cancels out. 3.0 miles 5280 feet 1 mile 12 inches 1 foot Train Track Unit Conversions How many inches are in 3.0 miles? 3.0 miles 5,280 feet 1 mile 12 inches 1 foot Inches are the only remaining unit ✔ Multiply all numbers on the top Divide all numbers on the bottom 3.0 x 5,280 x 12 1x1 = 190,080 inches = 1.9 x 105 inches (2 sig figs) More practice: Convert 1.40 x 10-6 g to mg Example Metric to Metric Conversion Problems Convert 2.79 x 105 mm to km Don’t try to convert directly from mm to km. Go to the base unit (m) first Conversion factors: 1,000 mm = 1 m; 1,000 m = 1 km 2.79 x 105 mm 1m 103 mm 1 km 103 m Kilometers are the only remaining units ✔ 2.79 x 105 = 2.79 x 10(5-3-3) 103 x 103 = 2.79 x 10-1 km (3 sig figs) More practice: Convert 3.4 x 103 cg to mg Example A person’s average daily intake of glucose (a form of sugar) is 0.0833 pound (lb). What is this mass in milligrams (mg)? (1 lb = 453.6 g.) A metric conversion is needed to convert grams to milligrams (1 mg = 1 × 10−3 g) (Or we could write: 1,000 mg = 1 g) Either Conversion factor will work 0.0833 lb 453.6 g 1 lb 103 mg 1g = 37,784.88 mg = 3.78 x 104 mg (3 sig figs) Example 2-D Conversion Problems (Unit1/Unit2) Convert 70.0 miles/hour to m/s We convert one unit at a time, followed by the other Conversion factors: 1 mile = 1,609 meters; 1 hour = 60 min; 1 min = 60 sec *Note: to cancel out hours (on bottom) it must appear again on the top 70.0 miles 1 hour 1609 meter 1 mile 1 hour 60 min 1 min 60 sec Meter/sec are the only remaining units ✔ 70.0 x 1,609 x 1 x 1 1 x 1 x 60 x 60 = 31.286 m/s = 31.3 m/s More practice: Convert 3.4 kg/L to g/mL 2-D Conversion Problems (Unit#) Example Convert 2.5 x 10-5 m3 to mm3 Conversion factors: 1 m = 1,000 mm 1 m3 ≠ 1,000 mm3 1 m3 = (1,000)3 mm3 ✔ 1. Write the 1-D units first (1m = 103m) 2. Add exponent to entire conversion factor 3 2.5 x 10-5 m3 1000 mm 1m = 2.5 x 10-5 m3 109 mm3 1 m3 2.5 x 10-5 x 109 = 2.5 x 104 mm3 More practice: Convert 34 yd2 to ft2 Example Convert 2-D Conversion Problems # (Unit ) 6.70 x 103 ft2 to inches2 Conversion factors: 1 ft = 12 in 1 ft2 ≠ 12 in2 1. Write the 1-D units first (1ft = 12 in) 1 ft2 = 122 in2✔ 6.70 x 103 ft2 12 in. 1 ft Alternate 2nd Method 2. Write the same conversion factor again until they cancel 12 in. 1 ft = 9.65 x 105 in2 More practice: Convert 34 yd3 to ft3 Example An average adult has 5.2 L of blood. What is the 3 volume of blood in m ? 1 mL = 1 cm3 5.2 L 103 mL 1L 1 cm3 1 mL 1 m3 1003 cm3 = 5.2 x -3 10 3 m Example 2-D Conversion Problem The circumference of the earth is approximately 2.49 x 104 miles long. If the speed of sound travels at 760 mph, how many days would it take a sound wave to circulate Earth’s circumference. Starting with length and velocity; needing time Conversion factor: 760 miles = 1 hour 2.49 x 104 miles 1 hour 760 miles 1 day 24 hours = 1.4 days More Practice Conversion problems • Convert 3.0 mL to ounces (33.8 oz = 1 L) • 42.0 km/h to ft/ms • 1.67 Mm to mm • 0.55 Acres to m2 (247 acre = 1 km2) • 2.35 x 1012 inches to cm (1 ft = 0.305 m) • 10.6 g/mm3 to kg/m3 • 3.50 x 104 mL to cL Review Unit Conversion & Significant Figures: Crash Course Chemistry #2 www.youtube.com/watch?v=hQpQ0hxVNTg Sample of Topics to Study • Metric base units • Derived unit • Using Prefixes • Significant Figures (+ math) • Scientific Notation (+ math) • % Error calculation •Accuracy • Precision • Dimensional Analysis