EDSE4275_Aspirin_EdSciPoster_Nov2015

Fluorescence of Acetylsalicylic Acid in an Aspirin Tablet
Research Question
By: Wacey Lym
Date: October 29, 2015
Scientific Research Poster Presentation
With the use of fluorescent spectrometry, determine the amount of acetylsalicylic acid (aspirin) in over-the-counter aspirin tablets.
Equipment/Materials
Mortar and pestle
1000 mL beaker
100 mL beaker
2 – 1000 mL vol. flasks
9 – 100 mL vol. flasks
3 – 50 mL vol. flasks
Pipets
Hot plate
Filter paper
Spectrofluorometer
Aspirin Tablets
Sodium hydroxide
Technique
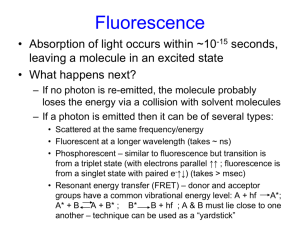
Fluorescence is the result of a three-stage process that occurs in certain molecules (generally polyaromatic hydrocarbons or heterocycles) called fluorophores or fluorescent dyes. Fluorescent probes enable researchers to detect particular components of complex biomolecular assemblies, such as live cells, with exquisite sensitivity and selectivity.
Background
Fluorescence is the emission of light by a molecule in an excited electronic state. The phenomenon of fluorescence in most cases represents the energy emitted when a substance returns from an excited or higher energy state to its normal or lower energy state. Two types of information may be obtained when the fluorescence emission is studied:
1. The intensity wavelength distribution, which is an indication of the electronic structures of the molecule.
2. The intensity at any wavelength where emission occurs which is an indication of the concentration of the fluorescent substance in the solution. It is this second aspect of fluorescence that we are mainly concerned with when using the total fluorescence method of analysis. In the total fluorescence measurement, the sample is irradiated with radiation of a known wavelength and the fluorescence emission which occurs over the complete wavelength range is measured.
Findings
The findings for this experiment are displayed in the table and graphs below.
Conclusions and Implications
The fluorescence accessory (spectrofluorometer) together with the Cary Concentration Application software can be used to successfully determine the amount of acetylsalicylic acid in a commercially available aspirin tablet.
Average % of acetylsalicylic acid in one aspirin tablet =
66.47%
±
3.35%
Fluorescence intensity is proportional to the number of fluorescing molecules in the sample, which would assume that a linear relationship exists between fluorescence intensity and concentration of the sample. Therefore, once the fluorescence is measured for each standard at each wavelength, a calibration curve can be constructed to determine the fluorescence vs. concentration. In practice however, this linearity is often absent and curvature is observed. A process called ‘self absorption’ is largely responsible for this curvature which occurs when the excitation and emission spectral envelopes overlap to a small extent. When this happens some of the emitted radiation is able to re-excite ground state molecules and is lost. The amount of self absorption increases with
©
File copyright Colin Purrington. You may use for making your poster, of course, but sites such as doctoc.com. If you have . insatiable need to post a template onto your own site, search the internet for a different template to steal. File downloaded from particles such as emission and absorption. http://colinpurrington.com/tips/academic/pos
Patterns
- different patterns may be observed at each of the terdesign.
causality in explanations of phenomena.
Literature Cited
"The Fluorometric Determination of Acetylsalicylic Acid in an Aspirin Tablet." Truman.edu. Truman State University, 30
Aug. 2006. Web. 28 Apr. 2014. <http://chemlab.truman.edu/CHEM322manual/pdf/fluorimetry.pdf>.
Miles, Corbin I., and George M. Schenk. "Fluorescence of Acetylsalicylic Acid in Solution and Its Measurement in
Presence of Salicylic Acid."
ACS Publications
. N.p., n.d. Web. 28 Apr. 2014.
<http://pubs.acs.org/doi/abs/10.1021/ac60288a032>.
"Using 73 Series to Determine Aspirin Concentration."
Using 73 Series to Determine Aspirin Concentration
. N.p., n.d.
Web. 28 Apr. 2014. <http://www.azom.com/article.aspx?ArticleID=10646>.
Fluorescence Process
HS-PS3-3. Design, build, and refine a device that works within given constraints to convert one form of energy into another form of energy.
This standard aligns with this project because students will use a form of energy to aid in calculations to determine another parameter. Students will measure the energy released through fluorescence and use that to determine concentrations and eventually lead to average mass percent in an aspirin tablet.
Energy and Matter
- changes of energy and matter in a system can be described in terms of energy and matter flows into, out of, and within that system.