Bohr Models and Lewis Dot Structures PowerPoint
advertisement

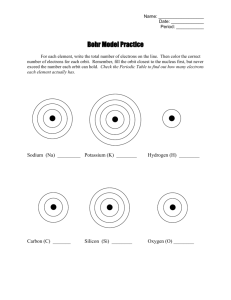
Introduction to Chemistry Bohr Models and Lewis Dot Structures Atoms • Basic unit of matter • Consists of: Electrons, Protons, & Neutrons Atoms Review • Consists of: Electrons, Protons, & Neutrons – Electrons: particles orbiting around nucleus with a negative charge – Protons: particles in the nucleus with positive charge – Neutrons: particles in the nucleus with no charge Element • a pure chemical substance consisting of one type of atom Atomic Number= Number of Protons or Electrons Symbol Name Atomic Mass= Number of Protons + Number of Neutrons I +1 II III IV V +3 +/-4 -3 +2 = element charges = Valence Electrons VI -2 VIII VII 0 -1 Periods and Groups • Periods go from left to • Groups go up and down right Bohr Models • Proposed by Niels Bohr in 1915 • Electrons orbit the nucleus in orbits that have a set size and energy. • The energy of the orbit is related to its size. • The lowest energy is found in the smallest orbit. Energy Levels • When two atoms approach each other their nucleus do not touch • Only electrons are involved in Chemical Reactions Bohr Diagrams • Find out which period (row) your element is in. • Elements in the 1st period have one energy level. • Elements in the 2nd period have two energy levels, and so on. Bohr Models RULE • • • • • • Show just electrons with energy levels 1st level holds 2 2nd holds 8 3rd holds 18 but is stable at 8 2-8-8 RULE!! Must fill an energy level to capacity before moving to the next energy level Bohr Diagrams Try the following elements on your own: a) b) c) d) e) f) H He O Al Ne K P: N: Valence Electrons • Electrons that are found on the outer most energy level. This determines the elements chemical properties Lewis Dot Structures • Named after Gilbert Newton Lewis • Show the bonding between atoms of a molecule, and the lone pairs of electrons that may exist in the molecule • Show only valence electrons (outer energy level) Lewis Dot Structures and Groups • Find out which group (columns) your element is in. • Elements in the 1st group have one valence electron. • Elements in the 2nd group have two valence electrons, and so on. Lewis Dot Try the following elements on your own: a) b) c) d) e) f) H He O Al Ne K