nomenclature
advertisement

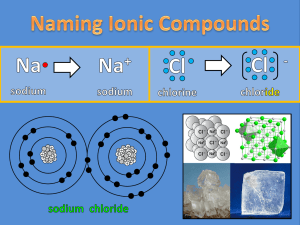
Binary Compounds Binary compounds contain only two elements. All binary compounds end in -ide. They are divided into two types, each of which has different rules for how to name them and write their formulas. IONIC = metal + nonmetal COVALENT = nonmetal + nonmetal Ionic Compounds Metal + Nonmetal Ionic compounds are held together by the opposite charge of the metal (+) and the nonmetal (-). The SUM of the charges of all the atoms must equal ZERO. Ionic Examples Na+1 and Cl-1 = NaCl sodium chloride Na+1 and O-2 = Na2O sodium oxide Ca+2 and O-2 = CaO calcium oxide Al+3 and Cl-1 = AlCl3 aluminum chloride Al+3 and O-2 = Al2O3 aluminum oxide Al+3 and N-3 = AlN aluminum nitride Transition Metals Transition metals can have different charges in different compounds. So … the name of the compound includes a roman numeral that indicates the charge on the transition metal. EXAMPLES: iron (II) chloride: Fe+2 and Cl-1 = FeCl2 gold (I) sulfide: Au+1 and S-2 = Au2S Transition Metals - cont. When you name a compound with a transition metal you must determine the charge on the metal. Remember … the sum of the charges of all atoms must be zero. So use the charge on the nonmetal to figure out the charge on the transition metal. EXAMPLES: CuF Cu + (-1) =0 Cu=+1 Mn2O3 2Mn + 3(-2) = 0 Mn=+3 manganese(III) oxide SnO2 Sn + 2(-2) = 0 Sn=+4 copper (I) fluoride tin (IV) oxide Covalent Compounds Nonmetal + Nonmetal Covalent compounds are held together by shared pairs of electrons. No ions are involved. Naming covalent compounds involves using a prefix system that directly indicates the number of atoms of each element in one molecule of the compound . Covalent Prefixes 1 = mono2 = di3 = tri4 = tetra5 = penta- 6 = hexa7 = hepta 8 = octa 9 = nona10 = deca- NOTE: Mono- is only used on the second element. In front of “oxide”, drop the “a” or “o” at the end of the prefix. Covalent Examples CO CO2 PCl3 P2I4 N2O S2F10 AsI2 B4C = = = = = = = = carbon monoxide carbon dioxide phosphorus trichloride diphosphorus tetraiodide dinitrogen monoxide disulfur decafluoride arsenic diiodide tetraboron monocarbide QuickTime™ and a decompressor are needed to see this picture. Polyatomic Ions Polayatomic ions are ions made from more than one atom, and usually more than one element. There is a single charge for the entire ion. They are ions, and are named as ionic compounds are. Parentheses are used when more than one of a polyatomic ion is needed. Examples of Polyatomic ions ammonium NH4+1 acetate C2H3O2-1 nitrite NO2-1 nitrate NO3-1 sulfate SO4-1 oxylate C2O4-2 phosphate PO4-3 hydroxide OH-1 cyanide CN-1 •Most polyatomic ions end in “-ate” or “-ite”. This separates them from binary compounds that end in “-ide”. •The positive polyatomic ion ammonium ends in “-ium”, like many metals. •Beware hydroxide and cyanide, which break the normal rules and end in “ide” but are polyatomic ions. Polyatomic Ionic Examples sodium acetate = Na+1 and C2H3O2-1 = NaC2H3O2 calcium nitrite = Ca+2 and NO2-1 = Ca(NO2)2 copper (II) phosphate = Cu+2 and PO4-3 = Cu3(PO4)2 manganese (II) sulfate = Mn+2 and SO4-2 aluminum cyanide = Al+3 and CN-1 = Al(CN)3 ammonium sulfide = NH4+1 and S-2 = (NH4)2S = MnSO4 Acids Aqueous acids have their own nomenclature, based on their negative ion. if ION ends in: then ACID is named: -ate -ic acid -ite -ous acid -ide hydro- & -ic acid Acid Examples HCl = hydrogen chloride -> hydrochloric acid HNO3 = hydrogen nitrate -> nitric acid HNO2 = hydrogen nitrite -> nitrous acid H2PO4 (aq) = phosphoric acid HI (aq) = hydroiodic acid H2SO3 (aq) = sulfurous acid H2C7H6O5 (aq)= citric acid HCN (aq) hydrocyanic acid =