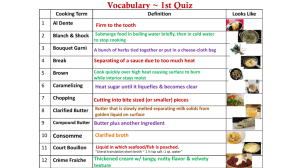
Research Proposal Title: Whipped cream as butter Name of
advertisement

Research Proposal Title: Whipped cream as butter Name of members: Lucy Antonio Magena Aquino Angela Candar Ma. Jeremee Chua Chapter I: Introduction: Butter is a product made from the solid components in milk (fat and protein). Although most often made from cow’s milk, butter can be made out of milk from sheep, goats, buffalo, or other mammals. Butter usually consists of approximately 80 percent fat, 15 percent water, and five percent protein. The small amount of protein in butter acts as an emulsifier allowing the water and fat to stay suspended in single-phase solution. The unique mixture of fats on butter allows it to stay solid at room temperature and melt at approximately 90 degrees Fahrenheit. Statement of the Problem: -What is fat? What does it have to do with butter? -How can cream be turned into water? -How does the heat affect the movement of particles? Formulation of the Hypothesis: -Cream can be made into butter -Churning is the process of shaking up the cream to produce butter -If cream consists of higher fat content then we can produce butter - When molecules are heated, they move faster because they have more energy. Significance of the study: Butter is affluent in Fat-Soluble Vitamins. Butter compromises Vitamins A, E and K2. Vitamin K2 can have dominant effects on health. It is well involved in calcium metabolism and a low consumption has been correlated with many serious diseases, including cardiovascular disease, cancer and osteoporosis. Butter is also associated with a lower risk of obesity Scope and limitations Our Investigatory Project is only focused on a simple butter and not in other butter such as Shea, margarine, etc. Also, it is focused on how the components of whipped cream can turn into butter. Definition of terms: Vitamin K2 -is a dietary supplement that may help reduce your risk of cancer and heart disease, and help support your immune system and bone health. Fat-Soluble Vitamins -any vitamin that is soluble in fats. Saturated fats -are found in animal products, including meat and whole milk dairy products, as well as certain plant oils like palm, palm kernel and coconut oils. Chapter II: Review of Related Literature Butter is one of the most highly concentrated forms of fluid milk. Twenty litres of whole milk are needed to produce one kilogram of butter. This process leaves approximately 18 litres of skim milk and buttermilk, which at one time were disposed of as animal feed or waste. Today the skim portion has greatly increased in value and is fully utilized in other products. Commercial butter is 80–82 percent milk fat, 16–17 percent water, and 1–2 percent milk solids other than fat (sometimes referred to as curd). It may contain salt , added directly to the butter in concentrations of 1 to 2 percent. Unsalted butter is often referred to as “sweet” butter. This should not be confused with “sweet cream” butter, which may or may not be salted. Reduced-fat, or “light,” butter usually contains about 40 percent milk fat. Butter also contains protein, calcium and phosphorous (about 1.2%) and fatsoluble vitamins A, D and E. Leah M. Melber, Alyce Hunter. "Integrating Language Arts and Social Studies: 25 Strategies for K-8 Inquiry" page 113. SAGE, 2009. ISBN 1-4129-7110-1. Butter – the everlasting delight of the gourmand, the faithful ally of the culinary arts, the constant symbol of good living. Through time and across the globe, butter has had a sacred quality. From the ancient Fertile Crescent to the present day, butter has symbolized the powerful, life giving and sacred, the good, the happy, the healthy and pure. It has sustained lives, cultures and civilizations for millennia. Michael Douma (editor). WebExhibits' Butter pages. Retrieved November 21, 2005. Chapter III: Methodology: A. Materials 3 cups Liquid measuring cup Heavy whipping cream Clean glass w/ lid and a tight seal, 1-qt. Stop watch Bowl Sealable plastic bags Permanent marker Table Procedure • Prepare a one-half cup of heavy whipping cream. Let it sit out at room temperature for about 5 hours • After sitting it out for 5 hours, pour it into a clean one quart glass jar. Put and close the lid tightly. • When you start shaking the jar, start the stopwatch or note what time it is. How does the heavy whipping cream change as you shake the jar? As the cream thickens (within a couple of minutes of when you start shaking), keep shaking the jar! • Shake the jar until butter forms. This could take between five to 20 minutes. Once you have shaken the jar enough, the liquid will suddenly separate from the butter. The butter will be a pale yellow lump, and the liquid will be milky. You'll probably hear the lump hitting the sides of the jar as you shake it. When the butter and liquid separate, stop shaking the jar and stop the stopwatch. • Carefully pour the liquid out of the jar. You can store the liquid and use it as buttermilk for other recipes. • Remove the lump of butter from the jar and place it in a bowl of cold water. Gently knead the butter to remove any extra liquid. Use your fingers to drain the liquid from the bowl. Rinse the butter two more times in this way. (If the liquid is not removed, the butter will go rancid faster.) • Transfer the butter into a small plastic bag and store it. • Clean the jar, its lid and the bowl. • Repeat the entire butter-making process as you just did but this time use one half cup of cold heavy whipping cream straight from the refrigerator (instead of room-temperature heavy whipping cream). Try to shake the jar similarly. Experimental Shaking time Room-temperature cream Controlled set-up Initial conditions Movement of particles Chapter IV: Results: Chapter V: Summary: As the cream is being shook, fat molecules get out of position and clump together and as they clump, butter forms. When the molecules are heated, they move faster because they do have more energy. The molecules in the room temperature cream moved faster that the chilled cream. Therefore, butter forms faster when they are in the room temperature. Conclusion: Arriving at our results and outputs, we came up that cream can be a substitute for butter and is effective. Recommendations: We would like to recommend this study because it teaches us the faster and cheaper way of making butter.