Physics Unit One and Two - Mill-Park
advertisement

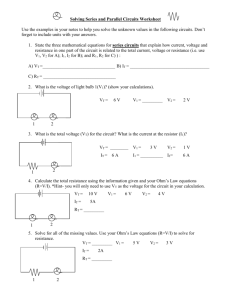
VCE PHYSICS STUDENT WORKBOOK UNIT 1 2013 NUCLEAR PHYSICS AND RADIATION ELECTRICITY DETAILED STUDY ONE Units 1 & 2 Physics - 2013 PLANNER TERM 1 1 Getting started 2y 3g UNIT ONE 5g 6y 7g Detailed Study One SAC 1 8y 9g Electricity 18 Mar 25 Mar 8g Revision and MidYear Exam 9y 10g Motion (continued) Chapters 3-5 11y 10 Jun 17 Jun 24 Jun Nuclear Physics and Radioactivity Fri 1st Feb 4 Feb 1y 2g Chapter 1 11 Feb 3y Electricity (continued) Chapters 2-3 TERM 2 18 Feb 25 Feb 4g 5y 4 Mar 6g SAC 3 11 Mar 7y UNIT TWO Motion 15 Apr 22 Apr 1g 2y TERM 3 15 Jul TERM 4 4y 3g Motion Continued Chapters 3-5 22 Jul 1g 2y Wave-like properties of light Chapters 6-8 7 Oct 29 Apr 14 Oct 6 May 13 May 20 May 27 May 3 Jun 4y 5g 6y 7g Detailed Study Two 8y 19 Aug 26 Aug 2 Sept 9 Sept 16 Sept 6y 7g Early Commence ment 8y 9g 10y 11g 11 Nov 18 Nov 25 Nov 2 Dec 9 Dec 16 Dec SAC 1 29 Jul 5 Aug 12 Aug 3g 4y Revision 5g 28 Oct 4 Nov SAC 2 21 Oct Exams 9g 10y Wave-like properties of light Chapters 6-8 Checklist for Unit One: Assignment Homework 1.1 Homework 1.2 Homework 1.3 SAC 1 Homework 2.1 Homework 2.2 SAC 2 Completed? Assignment Homework 3.1 Homework 3.2 Homework 3.3 Homework 3.4 Homework 3.5 Homework 3.6 SAC 3 Completed? SAC 1. My goal is … To understand: Finishing all these checkpoints will have you on course for an “A”. Don’t be surprised if you go into a SAC only completing half, and then get a “D”! o The Atom o Nuclear Radiation o Nuclear Decay o Half Life o Effects of Radiation on Humans To have practiced answering questions including: o Half life o Decay chains o Effects of radiation on humans SAC 3. My goal is … To understand: o Electrical Circuits o Electrostatics o Advanced Electric Circuits o Everyday Electricity To have practiced answering questions including: o Calculating resistance, voltage, current and power in circuits o Charges, electrons, forces, electric fields and energy in electrostatics o Calculating energy, power, resistance and current in everyday appliances Area of Study One: Nuclear Physics and Radiation The Atom Nuclear Radiation Nuclear Decay Half Life Effects of Radiation on Humans The Atom Three key figures: 1) Democritus: 2) J. J. Thompson: 3) Ernest Rutherford: Rutherford’s Gold Foil Experiment He concluded two things: 1) 2) Activity: Scale model of an atom Your teacher wants to make a scale model of an atom for the classroom. We want to use a tennis ball for the nucleus and marbles will be the orbiting electrons. If the size of a nucleus is r = 1x10-14m, and the electrons orbit at a distance of 1 x 10-10m; how far away do my electron marbles have to orbit the tennis ball? Extension: How small will the scale model nucleus have to be such that the entire scale model atom will fit in the classroom? The atom Draw the solar system model of the atom: Name Charge Mass (atomic units: a.u.) Proton Neutron Electron Particles that live in the nucleus are called _____________. Which particles from the above table are nucleons? Electromagnetic Force and Nuclear Force Protons have the same charge, and just like having two magnets with similar poles, protons repel each other How does the nucleus stick together? There is a force called the _____________________ It only works at _______________________________. Adding neutrons to a nucleus increases the __________________ force without increasing the ____________________ force. This is similar to adding a … Isotopes Isotopes are atoms of the same element… Isotopes have … Example: Carbon has 3 isotopes: Carbon -12, 13, and 14. All three isotopes have 6 protons. C-12 has _____ neutrons. C-13 has _____ neutrons. C-14 has _____ neutrons. Describing an atom: Z E A This is the __________________ for an atom Questions: Complete the following table Element 12 6𝐶 No. of protons No. of neutrons Name 143 Uranium 85 37𝑅𝑏 53 72 3 1𝐻 Radioisotopes Sometimes the nucleus of a particular isotope is __________, and it may undergo a … Every element heavier than ______________ is radioactive. Every element heavier than _____________________ has to be produced artificially. Nuclear Radiation Three types of radiation can be emitted in nuclear decay Symbol Name alpha beta gamma Made of Charge Mass Typical Energy Range in air Shielded by Ionizing ability Speed Isotopic Symbol Note: c = What is an electron volt? Atoms and electrons often contain very small amounts of energy if measured in Joules (~10-19J) It is the energy ____________ gains after being accelerated by _____ Questions 1) Convert into Joules a) 5eV b) 1keV c) 3MeV d) 0.2keV 2) Convert into eV a) 3.2x10-19J b) 6.4x10-14J c) 10-15J How is radiation detected? 1) When it touches the anode, it becomes an electrical pulse. The counter counts these electrical pulses 2) 3) Nuclear Decay Decay equations Example: Polonium-210 decays via alpha emmision. What product is formed? x= ________. y= _______. z= ________. Example: Carbon-14 decays via beta emission. Write a decay equation, and find the product formed. Note about beta decay: The product nucleus is called the __________________ Any further decay will lead to a ________________________ Questions: Write equations for the following 1) Uranium-238 decays via alpha emission 2) Francium-222 decays via beta emission 3) Polonium-214 decays via alpha emission 4) Carbon-12 emits gamma radiation 5) A new element is formed by adding a neutron to U-238 Half Life Activity is a measure: Questions 1) a) From the following graph, estimate the half life of the sample. Half life = b) Using this half-life, estimate at what time the sample will have an activity of 100 kBq 2) A hospital keeps a sample of Iodine-131 (I-131) for use in radiation therapy. It is noted that the activity of the sample has reduced by 75% in 17 days. What is the half life of I-131? 3) Carbon-14 is a radioisotope that is useful in obtaining the age of carbon containing materials (such as wood) up to about 50’000 years. Carbon-14 is a naturally occurring radioisotope that plants will absorb from the atmosphere, along with the non-radioactive isotopes carbon-13 and carbon-12. After the plant dies, it stops absorbing carbon, and the carbon-14 within the plant decays away. Carbon-14 decays through beta decay with a half-life of 5730 years. A team of archaeologists have discovered the remains of what looks to be a fire-pit used by primitive homo sapiens. They measure the amount of carbon-14 in samples taken from the fire-pit and find the measured ratio of carbon-14 to be approximately 6.25% of the ratio of atmospheric carbon-14. a) Approximately how many half-lives have elapsed since this sample was alive? b) Approximately how old is the sample? 4) Explain the joke… Effects of Radiation on Humans Isn’t always a bad thing! Has many uses But in large quantities it can be harmful… Which radiation is the most harmful? ___________ radiation has the highest ionizing ability. Ionizing: The radiation removes electrons from atoms in our cells/DNA. This causes the cells/DNA to mutate/die. However, alpha radiation is also … However… What type of radiation are these Fukishima workers being protected against? How do you know? Measuring radiation doses Absorbed Dose: The absorbed dose is the amount of radiation energy that has been absorbed per kilogram. Units: Dose Equivalent Different forms of radiation have different ionising abilities, and so cause varying amounts of damage to humans. As each type of radiation can affect tissue differently, the _______ _______________ is used compare accurately radiation effects. Units: Radiation Quality Factor Effective Dose eg. A person’s lungs would be more likely to develop cancer than the liver if they were both given the same amount of radiation. W= Σ= Body Part Ovaries/testes Bone Marrow Colon Lung Stomach Bladder Breast Liver Oesophagus Thyroid Rest of body Total Effects of radiation Short Term: Long Term: Weighting (W) Questions An 80kg tourist absorbs a gamma radiation dose of 200μGy during a return flight to England. Calculate the amount of radiation energy absorbed Calculate the dose equivalent that has been received During a medical procedure a patient receives a dose of gamma radiation. The organs which are affected include a bladder (5000Sv) and the ovaries (3000 Sv). Calculate the effective dose of radiation to which this woman has been exposed Area of Study Two: Electricity Electrical Circuits Electrostatics Advanced Electric Circuits Everyday Electricity Electrical Circuits Electrical Symbols: Device Wires crossed (not joined) Symbol Device Cell Wires joined Battery of cells Resistor AC supply Resistor Ammeter Filament Lamp Voltmeter Diode DC Supply Earth or ground Switch Symbol Draw: A) Two 1.5V cells connected to a lightbulb, and a switch B) A heating element (a resistor) connected to an AC power supply with a switch Are the following circuits different? Just because there are corners in a circuit diagram, doesn’t mean there has to be corners in the real circuit. This brings some confusion. Circuits that may appear __________ as diagrams, may actually be _____________. These are called _______________. Which of the following are equivalent circuits? Why Ohm’s Law Ohm’s Law… V= V= Units: I= Units: R= Units: ________________ obey Ohm’s Law _________________________________________. Their resistance is not constant Called ______________________________. Examples of Ohmic Conductors: Example of non-Ohmic Conductors: Questions: 1) A resistor draws a current of 1mA and has a voltage across it of 8V. Find the resistance 2) What current does a 1000Ω light bulb draw from a 24V power supply 3) You have an Ohmic resistor, which draws 75mA when connected to 12V power supply. You decrease the voltage to 5V, what current do you expect it to draw? 4) Which has more resistance? a. Fuse wire or power lines? b. Copper wire or Iron wire? The more resistance something has, the less current can go through it Resistance in a wire depends on: 1) 2) 3) 𝑅= ρ= Unit: Ωm L= Unit: m A= Unit: m2 Example Normal household wire has a diameter of 1.8mm, and is made from copper (ρ =1.7x10-8). What is the resistance of a 10m long section? Questions 1) A 100m long section of telegraph wiring has a diameter of 3.6mm and a resistivity of 2.8x10-8 Ωm. What is the resistance? 2) You have to design a household’s wiring system. Name three ways you could decrease the resistance as much as possible. 3) Why don’t all electricians try and use really thick wire all the time? Electrical Power Power is … Power = Questions 1) A bulb is connected to a 12V supply and draws 0.875A, what power is it using? 2) A toaster connected to the 240V mains supply uses 1kW of power. What current does it draw? 3) Which of the three identical bulbs in the picture shown is using the most power? 4) Which of these two bulbs (connected to different power supplies) is the brightest? Bulb 1 connected to 10V supply draws 0.8A of current. Bulb 2 connected to a 15V supply draws 0.4A. 5) A 50Ω heating unit is connected to a 20V power supply. What power does it use? Electrical Energy 𝐸= kWh is another unit of energy (Note it isn’t power!). 1kWh = What is electricity? 1e- = How many electrons in 1 C? What is current? 𝐼= What is voltage? 𝑉= Questions 1. How many electrons make up a charge of 1μC? 2. A) A hairdryer draws a current of 1.6A. What charge flows through the hair dryer every second? B) How many electrons flow through the hairdryer in 1 minute? 𝐸 3. Use the formulae 𝑉 = 𝑞 and 𝐼 = 𝑞 𝑡 , and substitute into P=VI, and simplify Electrostatics Law of conservation of charges: ____________________________ To make some negative charge you have to start with neutral atoms and split into an equal negative charge (electrons) and positive charge (remaining ion) Like charges __________ Unlike charges ___________________ An ___________ of electrons on something gives it a ______________ charge _______________ of electrons on something gives it a ____________ charge Van de Graff Generator How does it work? Conductors and Insulators Conductors: Insulators: Coulomb’s Law What caused the Al can to roll? 𝐹= k= Example: Two charges, with a charge equal to 1μC, are held 2m apart from each other. What is the force each charge exerts on each other? Examiners like to ask proportionality questions with Coulomb’ law Eg. Two charges, each with a charge of q, are positioned a set distance apart from each other. If the distance is doubled, how does the force change? Questions 1) A - 1μC and 6 μC are separated by 20cm. Calculate the force. 2) Two 1μC are separated by an unknown distance and experience a force of 3N. Calculate the distance separating the two charges. 3) A charge of q, and another of 2q are separated by a distance x. If the charge on the second is doubled to 4q, and the distance between them halves to x/2, how does the force change? Electric Fields The electrostatic force is invisible. However, as scientists, we have added lines around charges to help visualise this force Called ______________________________ Shows where a ________________ charge would fly off to if we dropped it into the field A ____________ charge would head in the _____________________. Where lines are ____________________, the field is ____________. Where lines are ____________________, the field is ____________. Field lines around a positive charge Field lines around a negative charge Some more complicated fields… 𝐹= E= . Units: Questions 1) Some small charged spheres are to be placed in an electric field which points downwards and has a strength of 5000NC-1. What force would be experienced by a charge of +5 μC and -0.6 μC ? 2) Draw the electric field you would expect around the three charges below -q +q -q 3) Draw the electric field you would expect around the two charges below +q -2q Advanced Electric Circuits Draw two different ways you could place two bulbs in a circuit with a battery Kirchhoff’s Laws Law 1: Law: “ Analogy What this means: Law 2: Law: Analogy: What this means: Resistors in Series 𝑅𝑇 = Eg Total resistance in the circuit? 6Ω 2Ω 3Ω 𝑅𝑇 = Resistors in Parallel 1 𝑅𝑇 1 𝑅𝑇 = = 𝑅𝑇 = The total resistance in a parallel circuit is always ____________than the ___________________. Sometimes examiners ask for the equivalent resistance (it’s the same thing as total resistance) Questions: Find the equivalent resistance of the following circuits: 1) 2Ω 1Ω 3Ω 2) 3Ω 4Ω Total resistance in hard circuits! Rules: : 12Ω 1Ω 6Ω Questions 3Ω 3) 4Ω 6Ω 4) 5) 5Ω 12Ω 5Ω 3Ω 3Ω 3Ω 10Ω 6Ω 2Ω 6) 3Ω 3Ω 6Ω 7) Draw a circuit with three resistors that has a total resistance of 5Ω. 8) Draw the above circuit differently. 9) Using a 1Ω, 2Ω, 3Ω, 4Ω and 5Ω resistor draw a circuit with total resistance = 6Ω One last note about voltage and current in circuits… The total VOLTAGE in a circuit is set by the _________________. However, the CURRENT drawn from this battery depends __________ _________________________________________ P.O.E. Draw two circuit diagrams with two bulbs (series and parallel) Which circuit will draw more current? ______________________________ Can you explain why? __________________________________________ __________________________________________________________ Circuit Measured Voltage Series Parallel Measured Current Which circuit uses the most power? ________________________________ ___________________________________________________________ Describe to someone else why one of the circuits draws more current: _______ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ Internal Resistance Resistance within the powerpack/battery. Uses some of the voltage up! Voltage without bulb = Voltage measured with bulb = Current = Voltage used in internal resistance Internal Resistance = Other sources of EMF EMF = ________ efficient _________ per square meter of sunlight energy Everyday Electricity We have only been dealing with DC electricity (_______________) Household electricity is ______ (___________________) Why? Power lines have a low resistance, but because electricity has to travel a large distance, this becomes an issue, and much power could be lost Current wants to flow from ___________ to ____________ just like it flows from __________ to __________ in a battery. Why do we have an earth wire? Imagine a fault in your toaster that touched the active wire to the casing. Then you touched the casing! Electric Shocks Our muscles move when they get small electrical shocks sent from our brain. Above __________ of electric current, and _____________________. A shock across the heart of ____________ for _________ = ______________________ Pracs – Unit One Index: Radiation and Nuclear Physics Detecting Radiation Decay chain Half Life Electricity Simple Circuits and Ohm’s Law Price of Electricity Electrostatics Series and Parallel Circuits Which bulb is the brightest? (Report) Electromagnets and bells EXPT: DETECTING RADIATION Aim Materials To investigate 2 different methods of detecting radiation: The Geiger counter and a cloud chamber. Part 1: Geiger Counter (Demo) 1. Your teacher will use the Geiger counter to attempt to detect radiation from an alpha source, a beta source and a gamma source. Write down your observations for the three sources: Alpha ………………………………………………………………………………………………… ………………………………………………………………………………………………………….. Clear plastic Cup Felt Plastercine/Blutak Iso-propanol Dry Ice Aluminium Oven tray (disposable) Geiger Counter Radioactive Sources Safety Beta ………………………………………………………………………………………………… …………………………………………………………………………………………………………. Gamma …………………………………………………………………………………………… ………………………………………………………………………………………………………….. 2. Your teacher will now attempt to block the three radiation sources with a piece of paper, a sheet of aluminium and a sheet of lead. First make a prediction: Follow teacher’s instructions if coming into contact with radioactive materials. Dry ice Iso-propanol What will stop: Alpha: ……………………………………………………………….. Beta: …………………………………………………………………. Gamma: ……………………………………………………………. 3. Write down your observations Alpha Beta Gamma Paper Aluminium Lead 4. The teacher will now take apart a smoke detector. What happens when the Geiger counter is brought near? __________________________________________________________________ 5. How might we figure out what type of radiation is being emitted? _____________________ _____________________________________________________________________________ _____________________________________________________________________________ 6. How does a smoke detector work? ______________________________________________ _____________________________________________________________________________ _____________________________________________________________________________ Part 2: Make a cloud chamber Method 1. Collect a plastic cup, felt, and scissors, plastercine/blue tack, oven tray. 2. Cut a circle in the felt, such that it will fit snuggly in the bottom of the cup. Attach the felt to the bottom of the cup with plastercine/blue tack 3. Soak the felt with iso-propanol, such that it is damp, but not dripping. 4. Cut a circle in your oven tray, such that it is slightly bigger than the top of the cup, and plastercine your cup upside down to this piece of metal. 5. Place your cloud chamber, with the metal facing down on top of a layer of dry ice Draw you observation: Bring a known radiation source close to the cloud chamber and describe what you see. …………………………………………………………………………………………………………………………………………………………… …………………………………………………………………………………………………………………………………………………………… …………………………………………………………………………………………………………………………………………………………… Place a magnet near your cloud chamber and describe any changes you see to the vapour trails …………………………………………………………………………………………………………………………………………………………… …………………………………………………………………………………………………………………………………………………………… …………………………………………………………………………………………………………………………………………………………… Describe where most of the radiation visible in the cloud chamber is coming from: …………………………………………………………………………………………………………………………………………………………… …………………………………………………………………………………………………………………………………………………………… …………………………………………………………………………………………………………………………………………………………… ACTIVITY: DECAY CHAINS Aim To investigate the transmutation of atoms. Part 1: Marshmallow Decay Chain Materials 8 Pink and 8 White Marshmallows per group Laptops 1. Pink Marshmallows are protons and white marshmallows are neutrons. 2. Take 6 protons and 6 neutrons and place together. Look up on the period table to find the name of this nucleus. Name: ______________________ Safety Don’t choke on your marshmallow… 3. Alpha particles are Helium nuclei. Make a separate alpha particle. 4. Some scientists have made new nuclei by bombarding atoms with alpha particles. Make your alpha particle and C-12 nuclei stick together. Look up in the period table what nucleus you have formed Name: ______________________ 5. Heavier elements like Uranium are radioactive. The spontaneous emission of an alpha particle is called alpha decay. Remove an alpha particle from your nucleus and write down the decay equation for this decay. You may eat your alpha particle. _________________________________________________________________________________ 6. Isotopes of an element have a different number of neutrons. Add two neutrons to your nucleus. What isotope have you formed? Name: ________________________ 7. Carbon-14 is a radioisotope and it undergoes beta decay. Change one neutron to a proton. What is the name of the new nucleus you have created. Name: ________________________ 8. Some heavy radioactive nuclei such as Ca-252 will undergo spontaneous nucleur fission, producing two nuclei of approximately the same size. Split your nucleus in two and write down the two products that you have created. Product One: _______________________ Product Two: ________________________________ Part Two: Decay Chain 1. Visit http://spice.duit.uwa.edu.au/samples/ast0197/ 2. Click on U-238. It decays via alpha emission. Click and drag to its possible product. The program will tell you if you are right or not. Keep decaying down until you get to a stable isotope. 3. Start another decay series at Am-241 and follow its decay series to a stable isotope. Copy down both of your decay series on the table below. Protons Neutrons 81 148 147 146 145 144 143 142 141 140 139 138 137 136 135 134 133 132 131 130 129 128 127 126 125 124 123 122 Tl-209 Tl-208 Tl-207 Tl-206 Tl-205 Tl-204 Tl-203 82 Pb-214 Pb-213 Pb-212 Pb-211 Pb-210 Pb-209 Pb-208 Pb-207 Pb-206 Pb-205 Pb-204 83 Bi-215 Bi-214 Bi-213 Bi-212 Bi-211 Bi-210 Bi-209 Bi-208 Bi-207 84 Po-218 Po-217 Po-216 Po-215 Po-214 Po-213 Po-212 Po-211 Po-210 85 At-219 At-218 At-217 At-216 At-215 At-214 At-213 At-212 At-211 At-210 86 Rn-222 Rn-221 Rn-220 Rn-219 Rn-218 87 Fr-223 Fr-222 Fr-221 88 Ra-229 Ra-228 Ra-227 Ra-226 Ra-225 Ra-224 Ra-223 Ra-222 89 Ac-229 Ac-228 Ac-227 Ac-226 Ac-225 Ac-224 90 Th-237 Th-236 Th-235 Th-234 Th-233 Th-232 Th-231 Th-230 Th-229 Th-228 Th-227 Th-226 91 Pa-237 Pa-236 Pa-235 Pa-234 Pa-233 Pa-232 Pa-231 Pa-230 92 U-240 U-239 U-238 U-237 U-236 U-235 U-234 U-233 U-232 U-231 U-230 93 Np-237 Np-238 Np-239 Np-240 Np-241 94 Pu-242 Pu-241 Pu-240 Pu-239 Pu-238 95 Am-241 ACTIVITY: HALF LIFE Aim Materials To investigate the transmutation of atoms. 2 dice each The decay of Unobtainium-280 1. Unobtainium-280 (Unb-280) decays via alpha decay. It decays every time a 6 is rolled. A roll of the dice represents 1 hour Safety No real safety concerns. 2. Each person has two dice and represents two Unobtainium atoms. Time Atoms remaining Draw a graph of your results with time on the x-axis A different isotope of Unobtainium (Unb-282) also decays via alpha decay, but here an atom of Unb282 will decay if an even number is rolled on a dice. Each roll represents 1 second. Time Atoms remaining Draw a graph of your results with time on the x-axis Use your results to predict the half-life of Unb-280 and Unb-282 Unb-280:__________________________ Unb-282:_________________________________ Why is it that we can not predict when a specific single atom might decay, but when we have millions and millions of atoms, we can accurately say when half of them have decayed? ……………………………………………………………………………………………………………………………………………………………. ……………………………………………………………………………………………………………………………………………………………. ……………………………………………………………………………………………………………………………………………………………. ……………………………………………………………………………………………………………………………………………………………. EXPT: SIMPLE CIRCUITS and OHM’S LAW Aim Materials To build a simple working electrical circuit. Measure current for different voltages and graph results Method 1. Take equipment listed to the right back to your desk 2. Plug in your power pack. Turn on at the wall, and turn voltage to 10V. 3. Set up the following circuit. Turn on your power pack to see if it works Power pack Wire x 4 Ammeters Multimeters 12V bulb Safety Electrical Safety 4. Change the voltage and write down your observation Observation: __________________________________________________ 5. We want to place an ammeter in the circuit to measure the amps. Ammeters are connected in series. Place the ammeter in the circuit as shown below A 6. Set your multimeter up to read DC Voltage. You teacher will help you. Voltage is measured across a load. Set you voltmeter to measure across the bulb as shown below A V 7. Change your multimeter between 0 – 12V, and measure the voltage and current every 2V. Make sure you read the right units off both the ammeter and voltmeter (ask your teacher if you are unsure) Current Voltage 8. Graph your results below (with current on the x-axis, voltage on the y-axis) 9. Calculate the gradient of your graph Gradient = Activity: The Price of Electricity Aim Materials To investigate the cost of running everyday electrical items. Method According to my electricity bill, electricity costs about 20c per kWh 1. Find the cost of electricity per J: 2. a) Investigate a microwave. How much power can it use? Microwave AA batteries (still in the packet) Kettle Access to solar panel info Watts Clever power meters. iphone charger Safety No real concerns b) Calculate the cost of heating a meal in the microwave for 5 minutes 3) a) Investigate a packet of 1.5V AA batteries. What voltage are these batteries? b) How many “milli-amp-hours” does each battery contain? [Milli-amp-hours, means how many hours it would run, if you were drawing 1mA] c) Multiply the number of milli-amp-hours by 3.6 to find “amp-seconds” d) Find the energy in the battery from the formula E = V x I x t e) Using this, and the price of electricity per J, how much should a battery cost? 4) a) How much power does a kettle use? b) Calculate the cost of boiling a jug of water (it takes 3 minutes) 5) a) Find the power that the solar panels on the roof of the science block are harnessing b) Assuming that the sun shines for an average of 5hours a day (this takes into account rainy days), how much money are we saving by using our own solar panels? 6) a) An iphone charger left plugged in to the wall will still some power. Use a “Watts Clever” power meter to measure how much energy is used when an iphone charger is plugged into the wall socket. b) How much does this cost, if it is left plugged in all year? Author’s Note: After writing this prac and working out all the values for myself, I raced around my house unplugging all our iphone chargers and laptops… EXPT: ELECTROSTATICS Aim To investigate the properties of charged insulators and their effect on other objects Method 1. Charge one of the rods (or a balloon) by rubbing it with one of the cloths. 2. Investigate what happens when you bring the charged rod close to small bits of ripped up paper Observation: ___________________________________________ Materials Plastic rods Balloons Wool/cloth Stand and clamp String Al Can Safety No real concerns 3. Investigate what happens when you bring the charged rod close to a horizontally suspended rod Observation: ___________________________________________ 4. Investigate what happens when you bring the charged rod close to a thin stream of running water Observation: __________________________________________________ 5. Investigate what happens when you bring the charged balloon close to an Aluminium can. Observation: __________________________________________________ 6. Investigate what happens when you bring the charged balloon close to another balloon. Observation: __________________________________________________ Explain you observations for four of the experiments above Small bits of paper: _________________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ Horizontal Rod: _________________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ Thin stream of water: _______________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ Al can: ___________________________________________________________________________ _________________________________________________________________________________ ________________________________________________________________________________ EXPT: SERIES AND PARALLEL CIRCUITS Aim Materials To build a series and parallel circuit and compare electrical properties between the two. Method 1. Take equipment listed to the right back to your desk Power pack Wire x 6 Ammeters Multimeters 12V bulb Safety 2. Plug in your power pack. Turn on at the wall, and turn voltage to 10V. Electrical Safety Part One: Series 3. Set up the following circuit. Turn on your power pack to see if it works A B C 4. Insert an ammeter into the circuit at points A, B and C. Record the current Current at point A ______________________ Current at point B ______________________ Current at point C ______________________ Observation/Conclusion: __________________________________________________ 5. With a multimeter measure the potential difference between the points A and B, B and C, and A and C Voltage between point A and B ______________________ Voltage between point B and C ______________________ Voltage between point A and C ______________________ Observation/Conclusion: __________________________________________________ Part Two: Parallel 6. Set up the following parallel circuit. A F B D C E 7. Insert an ammeter into the circuit at points A, B and C. Record the current Current at point A ______________________ Current at point B ______________________ Current at point C ______________________ Current at point D ______________________ Current at point E ______________________ Current at point F ______________________ Observation/Conclusion: __________________________________________________ 8. With a multimeter measure the potential difference between the points A and F, B and C, and D and E Voltage between point A and F ______________________ Voltage between point B and C ______________________ Voltage between point D and E ______________________ Observation/Conclusion: __________________________________________________ 9. Which bulbs were brightest: The bulbs in the series or parallel circuit? Why? 10. Sum up the rule for voltage around a series and parallel circuit in one sentence 11. Sum up the rule for current around a series or parallel circuit in one sentence EXPT: WHICH BULB IS THE BRIGHTEST? Aim Materials To investigate, build and calculate the circuit diagram below in order to prove which bulb is the brightest. THIS EXPERIMENT REQUIRES A REPORT HANDED IN. Your report should have the following format: 1) 2) 3) 4) Aim Circuit Diagram A description as to which bulb is the brightest. Proof as to which bulb is the brightest. Power pack Wire x 6 Ammeters Multimeters 12V bulb x 3 Safety Electrical Safety Notes: You may type up, or hand write your report. Hints: After building the circuit, measure the following coltages and currents: Voltage across bulb A:_______________________ Voltage across bulb B: _______________________ Voltage across bulb C: _______________________ Voltage across power pack: _______________________ Current at point (1): _______________________ Current at point (2): _______________________ Current at point (3): _______________________ For Part 4) Use these values above, as well as the formula for Power and Kirchhoff’s Laws, to work out the power in each bulb. 1 3 A C 2 B EXPT: ELECTROMAGNETS, BELLS AND BOMBS Aim To build an electromagnet, and with it make an electrical bell, a steady hand game, and if time persists, an alarm clock/bomb timer Materials Iron nail x 2 Power pack Wire Method Part One: Electromagnet and Bell Crocodile clip wire Metal strip Retort stand and clamps 1. Make an electromagnet by winding your wire tightly around your nail. 2. Set up the bell like the diagram shown below (and the demonstration at the front of the room) Safety Electrical Safety Clamps Electromagnet Metal Strip Nail Retort Stand 3. It may not be so obvious on the diagram above, but there is a small gap between the electromagnet nail, and the metal sheet. The metal sheet is resting lightly on the other nail. 4. The bell works in the following manner. The electromagnet is turned on, and receives a current. This attracts the metal sheet up, and it bends away from the bottom nail. As soon as it no longer touches the bottom nail, it will disconnect the circuit, turn off the electromagnet, and fall back down. This repeats the process all over again 5. Can you figure out how to connect up the bell to make it work? 6. Draw a circuit diagram of your working bell Part Two: Steady Hand Game 1. With the fencing wire, create a game, such that if your metal loop touches the edges, the bell goes off 2. Can you add in a light as well? 3. Draw a circuit diagram of your steady hand game. You can label your bell as: B Part Three: Alarm Clock (Extension) By wrapping aluminium foil around the hands of a clock, can you create an alarm that goes off at midnight? Describe how you did it: ____________________________________________________________ ________________________________________________________________________________ ________________________________________________________________________________ ________________________________________________________________________________ ________________________________________________________________________________ Skills for Physics 1 Scientific Notation Dealing with very big or small numbers using the scientific method Set One 1 )What is (in non scientific notation) a) 102 b)100 c)104 d)10-2 c)6 x 10-2 d)7 x 10-4 c) 100000 d) 0.1 c) 0.06 d) 0.007 e)10-4 2) Therefore, what is (in scientific notation) a) 2 x 102 b)5 x 104 3) Write these as powers of ten a) 100 b) 1000 e) 0.01 4) Put into scientific notation a) 200 b) 4000 5) Change out of scientific notation. a) 2.5 x 102 b)5.3 x 104 c)1.1 x 10-2 6) Put into scientific notation. a) 450 b) 1200 c)13 d)0.061 Set Two 1) Without a calculator, put these into scientific notation: a) 234 b) 20700 c) 65 d) 564000 2) Without a calculator, put these into scientific notation: a) 0.003 b) 0.3004 c) 0.000910 d) 0.0024 c) 1 x 103 d) 9.0009 x 105 c) 5.0 x 10-1 d) 8.401 x 10-3 3) Change out of scientific notation a) 3.5 x 106 b) 4.03 x 104 4) Change out of scientific notation a) 3.4 x 10-2 b) 3.004 x 10-4 5) Using your calculator, evaluate the following and keep the answers in scientific notation a) 40 x 354 b) 345 / 1245 e) 45 x 3104 / 3345201 c) 91 x 10003 6) Evaluate the following and write the answers in BOTH scientific notation and as standard number: a) 4.5 x 102 x 1.3 x 10-3 d) 452 b) 4.5 x 102 ÷ 1.3 x 103 c) 5.0 x 104 x 1.3 x 102 d) 7.4 x 102 ÷ 4.9 x 10-2 Set Three 1) Without a calculator, put these into scientific notation: a) 89 b) 50400 c) 2501 d) 80000 2) Without a calculator, put these into scientific notation: a) 0.0056 b) 0.11 c) 0.0008 d) 0.0305 3) Change out of scientific notation a) 7.6 x 103 d) 8.314 x 100 b) 1.00 x 104 c) 5.34 x 105 4) Change out of scientific notation a) 4.6 x 10-1 b) 4.074 x 10-3 c) 2.0 x 10-2 d) 3.9 x 10-4 5) Using your calculator, evaluate the following and keep the answers in scientific notation a) 604 x 235 b) 41 ÷ 4141 e) 22 x 345 ÷ 400559 c) 1000 x 100 6) Evaluate the following and write the answers in BOTH scientific notation and as standard number: a) 3.14 x 102 x 9.9 x 10-3 b) 9.1 x 101 ÷ 6.74 x 104 c) 3.0 x 104 x 1.0 x 101 d) 5.4 x 102 ÷ 5.2 x 10-3 d) 143 Skills for Physics 2 Algebra 1: Bedmas Brackets, Exponential, Division, Multiplication, Addition, Subtraction () x2 / Eg 6 + 2 x3 vs (6+2) x 3 Set One Evaluate the following (without a calculator) 1) 7+3x4 2) 6x2+1x3 3) 6/3+3 4) 6/(3+3) 5) 62 + 4 6) (6+4) 7) (7+3)x4 8) (7+3)x(6+4) 9) 7+3x6+4 10) 3x(6+4)2 – 1000/5 Set Two Evaluate the following (without a calculator) 1) 5+5 5 2) 52 +5 5 3) (5+5)2 5 4) 5 + 5 ÷ 5 − 5 5) 2 6 2 +3 6) 3 × 2 + 6 + 4 × 6 ÷ 2 − 12 × 2 7) 1 (20 − 2 12)2 8) 21 ÷ 3 + 7 9) 21 ÷ (3 + 7) x + - 6×106 10) 3×103 Skills for Physics 3 Algebra 2: The equation When answering questions in physics, we need to do everything in the correct order, so we can get full marks! That order is: 1) 2) 3) 4) 5) Write out the equation (with the symbols – not the numbers yet) Rearrange the equation if necessary Substitute the numbers in Dont forget the units! Calculate the answer (and don’t forget the units again!) For example: Given that the speed of a car is 10ms-1, and it travels for 20s, how far does it travel? 1) Write out the equation 𝑠= 𝑑 𝑡 2) Rearrange if necessary 𝑑 𝑡 ×𝑡 ×𝑡 𝑠×𝑡 =𝑑 𝑠= 3) Substitute the numbers in: 𝑑 = 10 × 20 4) Don’t forget the units! 𝑑 = 10𝑚𝑠 −1 × 20𝑠 5) Calculate the answer (don’t forget the units) 𝑑 = 200𝑚 Set One: 1) If the resistance of a lightbulb is 200Ω, and the current flowing through it is 0.1A, what is the voltage (V=IR) 2) What is the speed of a car that travels 1200m in 40 seconds? (s=dt) 3) If the voltage measured across a lightbulb is 12V and the current flowing through it is 0.001A, what is the resistance (V = IR) Set Two 1 1) Kinetic Energy is given by the formula = 2 𝑚𝑣 2 . Given a car is moving at 10ms-1 and It has a mass of 500kg, what is the kinetic energy of the car 2) Power of a light bulb is P=VI. Given the voltage is 240V and the current is 0.4A, what is the power? 3) Work is equal to force time distance (W = Fd). Given the work of a man pushing a box is 500J, and the distance is 10m, what is the force he is exerting? Skills for Physics 4 Algebra 3 – Rearranging simple equations. Given equations, such as s= d/t, we are often asked to calculate distance or time instead of speed. Now, we could either: remember all three equations (s=d/t, d = st, t = d/s) or remember the formula triangle, or we could just remember one formula (s=d/t) and learn how to rearrange formulas. In the long run this last option is by far the easiest! Let’s practice some simple rearranging. I like to think of the equation as balance. As long as I do the same thing to both sides of the equation, the equation will stay balanced. For example: 𝑥+2=5 +10 + 10 𝑥 + 12 = 15 This is still a valid equation! Adding ten to both sides wasn’t very helpful, but it still kept the equation balanced. Can you figure out what x is equal to? X = 3, again, you can find this from both equations. But we needed to do something to the above equation that was helpful. Like isolating the x by itself. Let’s do that by subtracting 2 from both sides 𝑥+2=5 −2 −2 𝑥=3 Now that was helpful! Lets now try a harder one, where multiplication is involved. 5𝑥 = 15 If your smart you might see that x must equal 3 in this case. Again we can do what ever we like to the equation as long as we do it to both sides 5𝑥 = 15 ×2 ×2 10𝑥 = 30 The equation is still balanced (the solution is still x=3), but multiplying by 2 wasn’t that helpful. Again, let’s think what we have to do to isolate the x. At the moment its multiplied by 5, so lets divide it by 5 5𝑥 = 15 ÷5 ÷5 𝑥=3 Rearranging equations with algebra in them, we go through exactly the same process. That is 1) Figure out the letter you want to get by itself 2) Do whatever you want to an equation, as long as you do the same to both sides! For example: What is the formula for speed, given that 𝑑 = 𝑠𝑡? 𝑑 = 𝑠𝑡 ÷𝑡 ÷𝑡 𝑑 =𝑠 𝑡 Set One: Rearrange these equations to find the symbol indicated in brackets. 1) (d) 𝑊 = 𝐹𝑑 2) (t) 𝑑 = 𝑠𝑡 3) (V) 𝑃 = 𝑉𝐼 𝐹 𝑚 4) (F) 𝑎 = 5) (v) 𝜌 = 𝑚𝑣 Set Two: 1) (t) 𝑠 = 𝑑 𝑡 1 2) (t) 𝑑 = 2 𝑔𝑡 2 1 3) (m) 𝐸 = 2 𝑚𝑣 2 4) (R) 𝑃 = 𝑉2 𝑅 5) (a) 𝑣 2 = 𝑢2 + 2𝑎𝑑 Skills for Physics 5 Unit prefixes Sometimes length measurements are made in metres (m). Sometimes they are made in centimetres (cm), millimetres, (mm), nanometres (nm) or kilometres (km). The words before the metres in all of these units are called prefixes Prefixes can be used with any other unit. They are used because it is makes more sense to talk about 3mm instead of 0.003m. The following table has the standard prefixes that we will use. The first column is the name. The second is the symbol. The third column is its value. The fourth column is also its value (but in scientific notation). Units means the unit, but without any prefix, eg grams or metres or amps. Prefix Name Mega Kilo Unit Milli Micro Nano Symbol M k Value 1’000’000 1’000 1 0.001 0.000001 0.000000001 m µ n Value 106 103 1 10-3 10-6 10-9 The “Value” columns tell us how this prefix relates to the unit. Eg, when talking about “milli” 1 milli metre = 0.001m 1 mega metre = 106 m or 1’000’000 You have to remember the golden rule with prefixes: Use the formula × 𝒘𝒂𝒏𝒕 𝒈𝒐𝒕 For example. Change 3km to m. So we WANT km, and we have GOT the unit. From the table 1km = 1000m 3 × 𝑤𝑎𝑛𝑡 1000 =3× = 3000𝑚 𝑔𝑜𝑡 1 Example: Change 5m to millimetres. We WANT mm,, we have GOT m. From the table 1mm = 0.001m 5𝑚 × 0.001 = 5000𝑚𝑚 Set One Change these prefixes to the units asked. 1) 4km to metres 2) 4.3km to metres 3) 0.67km to metres 4) 190km to metres 5) 55mm to metres 6) 65300 µm to metres Change these units to the prefixes asked 1) 3400m to km 2) 0.04m to mm 3) 290m to km 4) 67m to mm 5) 0.00704m to mm 6) 0.00000056m to µm Set Two Change these prefixes to the units asked 1) 120mA to A 2) 3.4kΩ to Ω 3) 65kg to g 4) 53000µm to m 5) 45keV to eV Change these units to the prefixes asked 1) 45000J to MJ 2) 0.00000065m to nm 3) 800W to kW 4) 0.02s to ms 5) 500000eV to keV Conceptual Understanding Procedures (Monash University) Physics Unit One and Two Physical Constants and Useful Formulae Physical Constants and Prefixes: Speed of light: 𝑐 = 3 × 108 𝑚𝑠 −1 𝑒𝑙𝑒𝑐𝑡𝑟𝑖𝑐 𝑐ℎ𝑎𝑟𝑔𝑒 = 1.6 × 10−19 𝐶 Acceleration due to gravity: 𝑎 = −9.8𝑚𝑠 −2 Radiation Alpha Beta Gamma Mega kilo centi milli micro nano Mega Quality Factor 20 1 1 M k c m μ n M 106 103 10-2 10-3 10-6 10-9 106 Useful Formulae: Nuclear and Radiation: Absorbed Dose 𝐴𝑏𝑠𝑜𝑟𝑏𝑒𝑑 𝐷𝑜𝑠𝑒 = 𝐸𝑛𝑒𝑟𝑔𝑦 𝑚𝑎𝑠𝑠 𝐷𝑜𝑠𝑒 𝑒𝑞𝑢𝑖𝑣𝑎𝑙𝑒𝑛𝑡 = 𝐴𝑏𝑠𝑜𝑟𝑏𝑒𝑑 𝐷𝑜𝑠𝑒 × 𝑞𝑢𝑎𝑙𝑖𝑡𝑦 𝑓𝑎𝑐𝑡𝑜𝑟 𝐸𝑓𝑓𝑒𝑐𝑡𝑖𝑣𝑒 𝐷𝑜𝑠𝑒 = Σ(𝐷𝑜𝑠𝑒 𝑒𝑞𝑢𝑖𝑣𝑎𝑙𝑒𝑛𝑡 × 𝑊) Dose Equivalent Effective Dose Electricity Ohm’s Law 𝑞 𝑡 𝐸 𝑉 = 𝑞 𝑉 = 𝐼𝑅 Power 𝑃 = 𝑉𝐼 Electric Charge Electric Work 𝐼 = Electrical Energy 𝐸 = 𝑃𝑡 Resistors in series 𝑅𝑇 (𝑆𝑒𝑟𝑖𝑒𝑠) = 𝑅1 + 𝑅2 +⋯ 1 1 1 (𝑃𝑎𝑟𝑎𝑙𝑙𝑒𝑙) = + 𝑅𝑇 𝑅1 𝑅2 +⋯ Resistors in parallel Motion Constant Velocity Constant Acceleration Equations of motion for constant acceleration Equations of motion for constant acceleration Equations of motion for constant acceleration Equations of motion for constant acceleration Newton’s 2nd Law Δ𝑥 Δ𝑡 Δ𝑣 𝑎= Δ𝑡 𝑣 = 𝑢 + 𝑎𝑡 Potential Energy 1 𝐸𝐾 = 𝑚𝑣 2 2 𝐸𝑃 = 𝑚𝑔ℎ Mechanical Work 𝑊 = 𝐹𝑑 𝑣 2 = 𝑢2 + 2𝑎𝑥 Power 1 𝑥 = 𝑢𝑡 + 𝑎𝑡 2 2 Momentum 𝑝 = 𝑚𝑣 Impulse 𝐼 = 𝐹Δ𝑡 = Δ𝑝 𝑣= 𝑥= 𝑢+𝑣 𝑡 2 Kinetic Energy 𝑃= 𝐸 𝑡 𝐹 = 𝑚𝑎 Waves Wave equation 𝑣 = 𝑓𝜆 Snell’s Law 𝑛1 𝑠𝑖𝑛𝜃1 = 𝑛2 𝑠𝑖𝑛𝜃2 Periodic table of the elements 1 H 1.0 2 He 4.0 Hydrogen Helium 3 Li 6.9 4 Be 9.0 79 Au 197.0 Lithium Beryllium 11 Na 23.0 12 Mg 24.3 Sodium Magnesium 19 K 39.1 20 Ca 40.1 21 Sc 44.9 22 Ti 47.9 23 V 50.9 24 Cr 52.0 25 Mn 54.9 26 Fe 55.9 27 Co 58.9 28 Ni 58.7 29 Cu 63.6 30 Zn 65.4 Potassium Calcium Scandium Titanium Vanadium Chromium Manganese Iron Cobalt Nickel Copper 37 Rb 85.5 38 Sr 87.6 39 Y 88.9 40 Zr 91.2 41 Nb 92.9 42 Mo 95.9 43 Tc 98.1 44 Ru 101.1 45 Rh 102.9 46 Pd 106.4 47 Ag 107.9 Rubidium Strontium Yttrium Zirconium Niobium Molybdenum Technetium Ruthenium Rhodium Palladium 55 Cs 132.9 56 Ba 137.3 57 La 138.9 72 Hf 178.5 73 Ta 180.9 74 W 183.8 75 Re 186.2 76 Os 190.2 77 Ir 192.2 78 Pt 195.1 Caesium Barium Lanthanum Hafnium Tantalum Tungsten Rhenium Osmium Iridium 87 Fr (223) 88 Ra (226) 89 Ac (227) 104 Rf (267) 105 Db (268) 106 Sg (269) 107 Bh (270) 108 Hs (269) 109 Mt (278) Francium Radium Actinium Rutherfordium Dubnium Seaborgium Bohrium Hassium Meitnerium atomic number relative atomic mass Gold symbol of element 5 B 10.8 6 C 12.0 7 N 14.0 8 O 16.0 9 F 19.0 10 Ne 20.1 name of element Boron Carbon Nitrogen Oxygen Fluorine Neon 13 Al 27.0 14 Si 28.1 15 P 31.0 16 S 32.1 17 Cl 35.5 18 Ar 39.9 Aluminium Silicon Phosphorus Sulfur Chlorine Argon 31 Ga 69.7 32 Ge 72.6 33 As 74.9 34 Se 79.0 35 Br 79.9 36 Kr 83.8 Zinc Gallium Germanium Arsenic Selenium Bromine Krypton 48 Cd 112.4 49 In 114.8 50 Sn 118.7 51 Sb 121.8 52 Te 127.6 53 I 126.9 54 Xe 131.3 Silver Cadmium Indium Tin Antimony Tellurium Iodine Xenon 79 Au 197.0 80 Hg 200.6 81 Tl 204.4 82 Pb 207.2 83 Bi 209.0 84 Po (209) 85 At (210) 86 Rn (222) Platinum Gold Mercury Thallium Lead Bismuth Polonium Astatine Radon 110 Ds (281) 111 Rg (281) 112 Cn (285) 113 Uut (286) 114 Fl (289) 115 Uup (288) 116 Lv (293) 117 Uus (294) 118 Uuo (294) Darmstadtium Roentgenium Copernicium Ununtrium Flerovium Ununpentium Livermorium Ununseptium Ununoctium 58 Ce 140.1 59 Pr 140.9 60 Nd 144.2 61 Pm (145) 62 Sm 150.3 63 Eu 152.0 64 Gd 157.2 65 Tb 158.9 66 Dy 162.5 67 Ho 164.9 68 Er 167.3 69 Tm 168.9 70 Yb 173.0 71 Lu 175.0 Cerium Praseodymium Neodymium Promethium Samarium Europium Gadolinium Terbium Dysprosium Holmium Erbium Thulium Ytterbium Lutetium 90 Th 232.0 91 Pa 231.0 92 U 238.0 93 Np (237.1) 94 Pu (244) 95 Am (243) 96 Cm (247) 97 Bk (247) 98 Cf (251) 99 Es (252) 100 Fm (257) 101 Md (258) 102 No (259) 103 Lr (262) Thorium Protactinium Uranium Neptunium Plutonium Americium Curium Berkelium Californium Einsteinium Fermium Mendelevium Nobelium Lawrencium