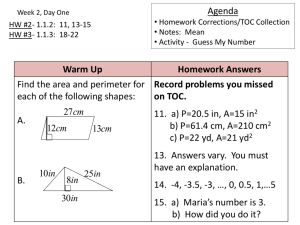
ToC CEDD - (S&I) Framework
advertisement

Transitions of Care Clinical Element Data Dictionary (ToC CEDD) February 2012 Release, Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework Transitions of Care CEDD Revision History Document Version 1.0 2.0 Date 12/1/11 2/29/12 Document Revision Description Includes A data elements approved by Initiative Includes B & C data elements approved by Initiative Page 2 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework Transitions of Care CEDD Table of Contents Overview ....................................................................................................................................................... 6 Audience ................................................................................................................................................... 6 Requisite Knowledge............................................................................................................................. 6 Introduction .................................................................................................................................................. 7 S&I Framework Background ..................................................................................................................... 7 ToC Initiative Overview ............................................................................................................................. 7 ToC CEDD Primer....................................................................................................................................... 8 ToC CEDD Origin........................................................................................................................................ 8 ToC CEDD Data Breakdown....................................................................................................................... 9 ToC Key Information Exchanges............................................................................................................ 9 ToC CEDD Objects ............................................................................................................................... 10 ToC CEDD Object Relationships .......................................................................................................... 12 ToC CEDD Data Elements .................................................................................................................... 13 ToC CEDD Vocabularies and Value Sets .................................................................................................. 15 Relevant Usage of the ToC CEDD ............................................................................................................ 16 ToC Scenario 1 User Story 1 ................................................................................................................ 17 ToC Scenario 1 User Story 2 ................................................................................................................ 17 ToC Scenario 2 User Story 1 ................................................................................................................ 18 ToC Scenario 2 User Story 2 ................................................................................................................ 18 Recommendations for S&I CEDD ................................................................................................................ 18 ToC CEDD Key Information Exchanges........................................................................................................ 19 ToC CEDD Key Information Exchange Summary ..................................................................................... 19 Consultation Request including Clinical Summary.............................................................................. 19 Consultation Summary........................................................................................................................ 20 Discharge Instructions......................................................................................................................... 21 Discharge Summary ............................................................................................................................ 22 ToC CEDD Objects ....................................................................................................................................... 24 ToC CEDD Objects in Detail ..................................................................................................................... 24 Admitting and Discharging Diagnoses ................................................................................................ 25 Allergies and Intolerances ................................................................................................................... 26 Page 3 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework Transitions of Care CEDD Behavioral Health History ................................................................................................................... 29 Care Team Members........................................................................................................................... 32 Consult(s) Assessments and Plan(s) Recommendations..................................................................... 33 Culturally Sensitive Patient Care ......................................................................................................... 34 Demographics ..................................................................................................................................... 36 Diet and Nutrition ............................................................................................................................... 37 Encounters .......................................................................................................................................... 40 Existence of Advance Directives ......................................................................................................... 42 Family History ..................................................................................................................................... 43 General Results ................................................................................................................................... 45 Goals ................................................................................................................................................... 46 History of Present Illness .................................................................................................................... 48 Immunization History.......................................................................................................................... 49 Invasive & Non-Invasive Procedures................................................................................................... 51 Medical Equipment ............................................................................................................................. 54 Medical History ................................................................................................................................... 55 Medications List .................................................................................................................................. 56 Operative Summary ............................................................................................................................ 61 Patient Contact Information ............................................................................................................... 62 Patient Information............................................................................................................................. 64 Patient Instructions ............................................................................................................................. 66 Payer Information ............................................................................................................................... 67 Physical Activity................................................................................................................................... 69 Physical Exam ...................................................................................................................................... 70 Primary Care and Designated Providers ............................................................................................. 71 Problems List ....................................................................................................................................... 73 Reason for Consult Request ................................................................................................................ 75 Review of Systems .............................................................................................................................. 77 Social History....................................................................................................................................... 78 Support Contacts ................................................................................................................................ 78 Page 4 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework Transitions of Care CEDD Surgical/Procedural History ................................................................................................................ 80 Women’s Health ................................................................................................................................. 81 Vital Signs ............................................................................................................................................ 82 Appendix A: CEDD Object Model Examples ................................................................................................ 85 Demographics Object Model Example.................................................................................................... 85 Consultation Request including Clinical Summary Object Model Example ............................................ 86 Consultation Summary Object Model Example ...................................................................................... 87 Discharge Instructions Object Model Example ....................................................................................... 88 Discharge Summary Object Model Example ........................................................................................... 89 Page 5 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework Transitions of Care CEDD Overview A data dictionary is a repository of data elements, their corresponding definitions, and attributes of the clinical information that is used in a clinical context. The dictionary lists the data elements and corresponding definitions that are needed to convey the clinical perspective in a manner that is understandable to a variety of stakeholders, including functional and technical experts. Additionally, these data elements support the electronic exchange of health information through a core set of unambiguously-defined data elements that promote semantic compatibility. The Transitions of Care (ToC) Clinical Element Data Dictionary (CEDD) represents the clinician perspective of clinical data required in care transitions to fulfill the ToC Use Case. In this document, the initial sections are to provide explicit guidance to stakeholders who may not have any exposure to the ToC initiative or the underlying mission of the ToC CEDD. Subsequent sections are focused on the more technical specifications recommended for the exchange of ToC CEDD Data Elements, including data types, references to HL7 CDA, and applicable value sets. Audience The intended audience for the ToC CEDD includes the following stakeholders: Stakeholder Providers and Specialists Care Coordinators Electronic Health Record (EHR) Vendors Personal Health Record (PHR) Vendors Usage of CEDD Provides a clinical perspective and view into care transition data relevant to providers and specialists. Ensures that in each care transition, the relevant clinical data that is needed by the care coordinator is available. Provides EHR vendors a view into the type of the clinical data needed to support each care transition. Provides PHR vendors a view into the type of patient-level data that care transitions produce and that may be requested from patients. Requisite Knowledge Readers are encouraged to have knowledge of the following concepts in order to best understand the intended usage of the ToC CEDD: Knowledge of Health Level Seven International (HL7) Clinical Document Architecture (CDA) R2. This is critically important as CDA served as the foundation for ToC CEDD. Knowledge of the ISO 21090 HL7 data type and CDA specifications. An overview of applicable data types is provided in the Usage of HL7/ISO Data Types section of this document Knowledge and functions of the Transitions of Care Initiative Page 6 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework Transitions of Care CEDD Introduction S&I Framework Background In support of the national objectives for healthcare reform, the Office of the National Coordinator for Health Information Technology (ONC) Standards and Interoperability (S&I) Framework has sponsored the development of harmonized interoperability specifications. These specifications are designed to support national health initiatives and healthcare priorities, including Meaningful Use, the Nationwide Health Information Network, and the ongoing mission to improve health care delivery, advance care coordination, and reduce costs to realize better population health. The S&I Framework is comprised of several initiatives, each focusing on a single challenge with a set of value-creating goals and outcomes to enhance the efficiency and effectiveness of healthcare delivery. The Transitions of Care (ToC) Initiative was among the first initiatives launched by the S&I Framework. The ToC Initiative focuses on empowering patients, engaging the clinician, and enabling health information exchange in support of national health initiatives. ToC Initiative Overview The purpose of the Transitions of Care initiative is to improve the exchange of core clinical information among providers, patients and other authorized entities electronically, in support of meaningful use and IOM-identified needs for improvement in the quality of care. The ToC Initiative is motivated by one very compelling question: What if every care transition was accompanied by an unambiguously-defined core set of high-quality clinical data? Key Functions of the ToC Initiative: Focus on core clinical content that could inform complete reconciled medication, problem, medication reaction, laboratory results, etc.; Build on existing standards to accelerate results; Work with the healthcare community to lower the implementation burden; and Guide decision-making based on the requirements of meaningful use and IOM-identified needs for improvement in the quality of care. Key Outputs of the ToC Initiative: Unambiguous definition of the core clinical elements that should be included in care transitions Definition of four key clinical Constructs that provide guidance on the exchange of information in the event of a patient care transition Agreement on a single standard in support of Meaningful Use requirements, which minimizes interoperability errors and streamlines patient care coordination Page 7 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework Transitions of Care CEDD Tools and resources to lower the barrier for implementation ToC CEDD Primer In addition to addressing the need for an unambiguously-defined core set of clinical data, the ToC CEDD is intended to serve as a logical overlay and neutral representation of the data needed to support care transitions. The value proposition inherent in the ToC CEDD is that it: a. Provides a view for clinicians into the type of data needed to support each care transition b. Provides implementers and vendors an idea of how to store and exchange that data c. Serves as a logical view of the common data model that underlies all care transitions In practice, it will manifest itself as physical data within an organization engaged in care transitions. The work on the ToC CEDD was guided by practicing clinicians and other implementers who were interested in creating a simple, easy-to-understand model for functional stakeholders to use. The ToC CEDD also draws heavily from best practices and models defined by several organizations supporting the S&I Framework mission, including: National E-Health Transition Authority (NEHTA) Federal Health Information Model (FHIM) HL7 Version 3 GE/Intermountain Healthcare Clinical Element Models (CEM) Quality Data Model (QDM) Specific sources of information were drawn from the existing work of these organizations to create ToC CEDD Objects and to help define the structure of the dictionary. It was not the intention in the development of the ToC CEDD to specifically adopt an information model already in use, nor to redefine existing information models – the objective was to leverage previous work to create a new type of representation specifically targeted to the requirements of clinicians who may not have a deep understanding of care transition data, its structure, and its flow. ToC CEDD Origin Throughout the development of ToC Initiative specifications, the need for a common information model became apparent during analysis of existing standards and barriers to electronic exchange of information. While common data elements existed between the ToC Selected Standard, HL7 Clinical Document Architecture (CDA), other applicable standards, and various health care information models, ambiguous definitions prevented accurate harmonization for an interoperable standard. To best demonstrate the complex relationships between data elements and objects, the Initiative initially pursued the development of a Clinical Information Model (CIM), which combined the traditional data dictionary model with a logical model. The resulting CIM was the harmonization of the clinical requirements for the ToC Use Case Scenarios, the CDA, and other applicable standards for a harmonized care transition information model. Page 8 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework Transitions of Care CEDD As an important output of the ToC Initiative, the CIM is best represented in the form of a data dictionary. The transition ensured a clear representation of data elements supporting the ToC Use Case, while maintaining the level of abstraction necessary to support various business needs. In congruence with this change in representation, the ToC CIM is now referred to as the ToC CEDD, but maintains the important modeling properties to best serve as an artifact for reuse and a tool for implementers. The following section outlines these modeling properties, as employed by the ToC CEDD. ToC CEDD Data Breakdown A complete ToC CEDD concept includes Key Information Exchanges, ToC CEDD Objects and ToC CEDD Data Elements. These terms may also collectively be referred to as the ToC CEDD Data Structure, a figurative term used to outline the dependencies between these three concepts. Figure 1 provides an overview of this CEDD Data Structure: Figure 1 - ToC CEDD Data Structure For clinicians, this section may be useful to help understand how the ToC CEDD is structured. Additional Object representations may be found in Appendix A to assist in understanding ToC CEDD data concepts. ToC Key Information Exchanges ToC CEDD Key Information Exchanges contain the clinical information to meet the functional requirements of the ToC Use Case defined actors. The ToC Use Case defines four clinical constructs represented through ToC CEDD Key Information Exchanges. Page 9 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework Transitions of Care CEDD Characteristic ToC CEDD Key Information Exchange Definition ToC Reference Description of the Characteristic A clinically-relevant name for this ToC Key Information Exchange. This name should be understandable to clinicians. A clinically-relevant definition of the ToC CEDD Object. References the ToC Use Case Scenario defining the Key Information Exchange The ToC CEDD Key Information Exchanges are groupings of ToC CEDD Objects, but for ease of understanding are highlighted in the ToC CEDD to provide implementers an origination point for ToC constructs to be implemented through the ToC CDA Consolidation Companion Guide. To assist in traceability, parent and child object designations for objects reflect relationships to the ToC Key Information Exchanges. Figure 2 depicts a rudimentary example of how a Key Information Exchange is structured. Key Information Exchange Discharge Summary Medications List Consult(s) Assessment(s) & Plan(s) Recommendations Existence of Advance Directives Immunization History Allergies and Intolerances Problems List Medical Equipment Demographics Figure 2 - Key Information Exchange ToC CEDD Objects are intended to be used primarily for requirements traceability, meaning that they are intended to map clinical data to the requirements of a use case. Because this representation of the ToC CEDD is CDA-based, the primary level of traceability is from the ToC CEDD to the ToC Key Information Exchanges defined in the ToC Use Case. ToC CEDD Objects A ToC CEDD Object represents a specific entity within a logical data model. Each ToC CEDD Object is designed to map to an underlying concept that is of some familiarity to practicing clinicians and specialists, and other stakeholders who may be involved in healthcare organizations. The ToC CEDD is specifically targeted to stakeholders involved in care transitions processes. Page 10 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework Transitions of Care CEDD A key differential with ToC CEDD Objects is that they are not tied to any specific underlying information model. Thus, as an example, a ToC CEDD Object is not tied to the HL7 Reference Information Model (RIM), although it may use concepts or terms that are similar to the RIM. As noted in the previous section, the independence of ToC CEDD Objects from an underlying standard provides the flexibility for ToC CEDD Objects to be reused in other contexts. For ToC CEDD Objects, several key pieces of information are defined to assist in understanding clinical meaning. ToC CEDD Object characteristics are summarized in the following table: Characteristic ToC Priority ToC CEDD Object Name Object Definition Examples and CDA ID References Description of the Characteristic The priority of this ToC CEDD Object. Please reference the ToC CEDD Priorities section to understand their application. A clinically-relevant name for this ToC CEDD Object. This name should be understandable to clinicians. A clinically-relevant definition of the ToC CEDD Object. Additional usage guidance may also be included in italics. Provides real-world clinical examples to provide context for usage as well as listing CDA-specific references for Document, Section, and Entry IDs ToC CEDD Priorities ToC CEDD Priorities were used to prioritize the development of the ToC CEDD and determined the key clinical information needed for exchange during care transitions within the scope of the ToC Use Case. These priorities have been reviewed by clinicians and other stakeholders involved in care planning and care transitions within healthcare organizations throughout the United States. The following table summarizes the ToC CEDD Priorities and their applicability: ToC CEDD Priority "A" "B" Description of Priority Core data exchanged with every transition of care These may be automated by the edge system (EHR) "A" objects have validated data models Required indicates that every clinical document created must have core objects NB subsets of categories of "additional" objects (e.g. several results from the hundreds that may be in the EHR database for a patient) can be added by the clinician end user to the Direct Message depending on the clinical circumstance. The variable objects are selectively added to prevent information overload by the recipient clinician (e.g. a recipient clinician receiving several hundred results for a patient following an extended hospital stay would lead to the recipient clinician being data overloaded and not caring for the patient as effectively as in the circumstances of receiving the selected 2 or three results that would be helpful to the PCP for efficient care and management of the patient). Selected "B" objects are either very frequently required in most transition of care circumstances (e.g. results) and/or are regularly captured in many EHR systems as Page 11 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework Transitions of Care CEDD ToC CEDD Priority "C" Description of Priority discrete data. Variable data needed by the end user in some transition of care circumstances Selected "C" objects are either less frequently required in most transition of care circumstances and/or are not currently captured in many EHR systems as discrete data It is important to understand that ToC CEDD Priorities served primarily to assist clinicians in building out the initial ToC Clinical Information Model (previously addressed in Origin of the ToC CEDD). Designation of priorities allowed development of specific objects and data elements to occur in a fashion to reflect the priorities of clinical information needs during a care transition instance. While priorities were initially assigned at both the object and data element level, they are utilized in this version of the ToC CEDD only at the object level, to provide traceability and for reference by implementers seeking to reuse ToC CEDD Objects in CDA documents. ToC CEDD Object Relationships ToC CEDD Objects are intended to capture a real-world clinical concept and are not intended to be represented as physical objects, meaning the representation of how data is stored within a physical data store. To convey relationships between specific entities, ToC CEDD Objects contain properties reflecting designation as a parent or child object. However, as noted in the Origin of the ToC CEDD, the abstract nature of the ToC CEDD prevents dependence on a rigid parent/child hierarchical structure, and data elements or objects may inherit attributes from more than one parent. Similarly, objects may incorporate specific attributes rather than all attributes of another object. These properties are further detailed in the following sections, and specific designations are listed within individual ToC CEDD Object summaries as applicable. Figure 3 depicts the parent-child relationship of ToC CEDD Objects and the related ToC CEDD Data Elements, using Admitting and Discharging Diagnoses as an example: Page 12 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework Transitions of Care CEDD Figure 3 - Admitting and Discharging Diagnoses - CEDD Data Concept Example ToC CEDD Data Elements A ToC CEDD Data Element is an attribute of a ToC CEDD Object. For ToC CEDD Data Elements, several key pieces of information are defined to assist in understanding clinical meaning: Characteristic Data Element Name Data Element Definition Clinical Example HL7/ISO Data Type CDA Reference Expected Value Set Description of the Characteristic A clinically-relevant name for this ToC CEDD Data Element. This name should be understandable to clinicians. A clinically-relevant definition of the ToC CEDD Data Element. Additional usage guidance may also be included in italics. Provides real-world clinical examples to provide context for usage. A possible data type that may be used to represent this ToC CEDD Data Element. They are aligned to the ToC Recommended Standard: HL7 CDA. Representation of the ToC CEDD Data Element through HL7 CDA. Identifies the section of an applicable value Stakeholders All All Providers/Specialists and Care Coordinators EHR Vendors and PHR Vendors EHR Vendors and PHR Vendors EHR Vendors and Page 13 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework Transitions of Care CEDD Characteristic Description of the Characteristic set made available through the S&I Framework CEDD Value Set Index. Stakeholders PHR Vendors Usage of HL7/ISO Data Types A core set of data types is needed to support the representation of the ToC CEDD. The reason for this is that the ToC CEDD is not based on any underlying information model, and thus has to use a set of data types from a source to represent data logically. The ToC CEDD adopted the ISO/HL7 data types that are commonly used as part of the HL7 RIM and the HL7 CDA. The ISO 21090 Healthcare Data Type Standard provides a set of data type definitions for representing and exchanging basic concepts that are encountered in healthcare environments, and specifies a collection of healthcare-related data types suitable for use in a number of healthcare-related information environments. This standard is a culmination of a large-scale joint effort among standards bodies, such as HL7 and ISO, and has been reviewed by experts in the field. It should be noted that several of the data types referenced in this list are specific to the HL7 CDA. As noted, a user of the ToC CEDD should have basic knowledge of the CDA. The following table provides an overview of the data types used in the ToC CEDD: ISO/HL7 Data Type AD Description Usage Address Used to capture a physical address Used to capture phone numbers and email addresses Used to capture the name of a person TN Telephone Number PN Person Name Coded Element with formatted values CF ED Encapsulated Data BAG Bag SET Set HIST History LIST IVL IVL_TS CS List Sequence Interval Interval – Timestamp Coded – Simple Value PQ Physical Quantity CE Coded Element Similar to CE but with formatted values Used to capture text and multimedia that may be included in a care transition Used to capture a an unordered, multiple collection of things Used to represent an unordered collection type that stores unique elements Used to capture historical items about something or set of things Used to store ordered, non-unique elements Used to capture an interval of things Used to capture an interval of time Used to capture a simple set of codes Used to capture information about quantities, through a value and a unit of measure Used to capture a specific coded element or set of coded elements Page 14 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework Transitions of Care CEDD ISO/HL7 Data Type Description BL Boolean DATE Date Used to capture Boolean information (i.e. true/false, yes/no) Used to capture a date II Instance Identifier Used to identify a unique instance of some thing INTEGER Integer EN Entity Name Used to capture a number Used to capture the name of an individual or organization Usage Structure Data Type In addition, the ToC CEDD defines a Structure data type. This data type is used in those cases where the data assembled might be another object or discrete set of data that is assembled somewhere else (outside the scope of the ToC CEDD). It is important to note that many of the ToC CEDD Data Elements can potentially be expressed using multiple data types. This is one of the foundational principles of the ToC CEDD itself; it is not meant to be prescriptive or to require conformance, it is simply meant to serve as a tool to represent the perspective of the clinician. As such, design decisions surrounding a Structure data type can be made by implementers and vendors, depending on the base derived data type within their environment. Example: The Physical Activity Assessment Data Element has a data type of LIST (List Sequence), but data types of CE (Coded Entries), SET, or ED (Encapsulated Data) may also be assigned to best fit the needs of an organization. ToC CEDD Vocabularies and Value Sets ToC CEDD Objects use terms that are drawn from several code systems. These controlled vocabularies are defined in various supporting specifications, and may be maintained by other entities, as is the case for the LOINC® and SNOMED CT® vocabularies. As a general rule, the vocabularies and value sets defined in the CDA are inherited by ToC CEDD Objects and ToC CEDD Data Elements. Specific value sets are available through the S&I Framework CEDD Value Set Index on the S&I Framework CEDD Value Set Index wiki page. Value sets are listed at ToC CEDD Data Element level and indicate applicable sections within the S&I Framework CEDD Value Set Index. Overall, the ToC CEDD aligns to the recommendations of the Health IT Standards Committee (HITSC) for data elements that have associated vocabulary/code set requirements. The following table captures the high-level recommendations from this committee: ToC CEDD Object Physical Exam Family History ToC CEDD Data Elements Component Observation Component Vocabulary Recommendation LOINC SNOMED-CT LOINC Page 15 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework Transitions of Care CEDD ToC CEDD Object Active Medication List Procedures Problem List Equipment Culturally Sensitive Patient Care Payer Information ToC CEDD Data Elements Response Active Medication List Procedure Problem Equipment Race Gender Language Primary Payer Information Secondary Payer Information Vocabulary Recommendation SNOMED-CT RxNORM SNOMED-CT SNOMED-CT SNOMED-CT PHIN-VADS HL7 ISO 639-2 ASC X12 ASC X12 For the ToC CEDD, additional vocabularies and value sets can be reused from the National Library of Medicine (NLM) mappings and subsets available through the Unified Medical Language System (UMLS). Example: An implementer may wish to implement a discharge summary using an existing vocabulary already implemented within their environment. The ToC CEDD Object Problem List does not exclude the use of this vocabulary, so long as an accurate mapping exists back to the SNOMED-CT recommendation provided by the HITSC Vocabulary Task Force. Relevant Usage of the ToC CEDD Associated usage diagrams for the ToC CEDD are provided to give context to implementers and clinicians about different usage scenarios for the ToC CEDD. This section specifically highlights the four ToC Use Case Scenarios outlined in the S&I Framework Transitions of Care Use Case. Please note all diagram references to “CIM Objects” are in reality to “CEDD Objects.” Page 16 of 89 Version 2.0 ToC Scenario 1 User Story 1 ToC Scenario 1 User Story 2 ToC Scenario 2 User Story 1 ToC Scenario 2 User Story 2 Recommendations for S&I CEDD ToC CEDD Data Elements with an “A” priority designation were accepted for inclusion in the S&I CEDD in December 2011 and comprise the January release of the S&I Framework CEDD. ToC CEDD Data Elements included within this version of the ToC CEDD for recommendation to the S&I Framework CEDD are highlighted in light red to indicate consensus by ToC, but are recommended for consensus by the S&I CEDD WG, and subsequent inclusion in the S&I Framework CEDD. For more information on the recommendations process, please reference the S&I CEDD Cross-Initiative WG wiki. Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD ToC CEDD Key Information Exchanges ToC CEDD Key Information Exchange Summary The following table summarizes each of the ToC CEDD Key Information Exchanges in alphabetical order. This table may be used to reference ToC CEDD Objects comprising ToC CEDD Key Information Exchanges. It is important to note that ToC CEDD Key Information Exchanges are ToC CEDD Objects, but for ease of understanding, have been highlighted in this section to provide implementers an origination point for ToC constructs to be implemented through the ToC CDA Consolidation Companion Guide. Certain ToC CEDD Objects may not be listed as part of a ToC CEDD Key Information Exchange, but applicable relationships are indicated within individual ToC CEDD Objects. Other ToC CEDD Objects that are not listed as part of a ToC CEDD Key Information Exchanges or as other related objects are for future development by any interested party. Consultation Request including Clinical Summary Key Information Exchange Consultation Request including Clinical Summary Consultation Request including Clinical Summary Key Information Exchange Definition This information exchange would include a standard set of data including demographic information, active reconciled medication list (with doses and sig), allergy list and problem list. The Clinical Summary may also contain variable data relevant to the context of the request. In addition, this document also includes a PCP-selected referral-specific variable dataset. ToC Reference ToC Scenario 1 User Story 2 ToC Scenario 2 User Story 2 Consultation Request including Clinical Summary Parent Object ToC Priority Child Objects A Allergies and Intolerances B Consultant(s) Assessment(s) and Plan(s) Recommendations A Demographics B Encounters A Existence of Advance Directives B Family History B General Results B History Present Illness Page 19 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Consultation Request including Clinical Summary Parent Object ToC Priority Child Objects A Immunization History B Invasive and Non-Invasive Procedures C Medical Equipment B Medical History A Medications List A Payer Information B Physical Exam B Problems List B Reason for Consult Request B Social History B Surgical/Procedural History B Vital Signs Consultation Summary Key Information Exchange Consultation Summary Consultation Summary Key Information Exchange Definition This information exchange will include a standard data set including demographic information, active reconciled medication list (with doses and sig), allergy list and problem list. This information exchange would also contain variable data relevant to the context of the request. ToC Priority A B A B A ToC Reference ToC Scenario 1 User Story 2 Consultation Summary Parent Object Child Objects Allergies and Intolerances Consult(s) Assessment(s) and Plan(s) Recommendations Demographics Encounters Existence of Advance Directives Page 20 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD ToC Priority B B B A B C B A A B B B B B B Consultation Summary Parent Object Child Objects Family History General Results History Present Illness Immunization History Invasive and Non-Invasive Procedures Medical Equipment Medical History Medications List Payer Information Physical Exam Problems List Reason for Consult Request Social History Surgical/Procedural History Vital Signs Discharge Instructions Key Information Exchange Discharge Instructions Discharge Instructions Key Information Exchange Definition This information exchange would include a standard data set including demographic information, active reconciled medication list (with doses and sig), allergy list and problem list. Discharge Instructions also contains dataset relevant to the Discharge Summary/Discharge Instructions context which includes follow-up/plan of care. ToC Priority A ToC Reference ToC Scenario 1 User Story 1 ToC Scenario 2 User Story 1 Discharge Instructions Parent Object Child Objects Allergies and Intolerances Page 21 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD ToC Priority B A A A C A B Discharge Instructions Parent Object Child Objects Consult(s) Assessment(s) and Plan(s) Recommendations Demographics Existence of Advance Directives Immunization History Medical Equipment Medications List Problems List Discharge Summary Key Information Exchange Discharge Summary Discharge Summary Key Information Exchange Definition This information exchange would contain a standard set of data surrounding a discharge, and discharge context-relevant data, which is determined by the discharging provider organization in accordance with local policy, regulations and law. The receiving provider through its EHR system may determine how to incorporate and present the Discharge Summary document. The Discharge summary should always include a basic set of information on the discharge that might also include content for the Discharge Instruction as well as the Discharge Summary. Discharge summary content examples include demographic information, active reconciled medication list (with doses and sig), allergy list, problem list, and reason for admission. ToC Priority B A B B A B ToC Reference ToC Scenario 2 User Story 1 Discharge Summary Parent Object Child Objects Admitting and Discharging Diagnoses Allergies and Intolerances Consult(s) Assessment(s) and Plan(s) Recommendations Diet and Nutrition Demographics Encounters Page 22 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD ToC Priority A B B B A B C B A A B B B B B B Discharge Summary Parent Object Child Objects Existence of Advance Directives Family History General Results History Present Illness Immunization History Invasive and Non-Invasive Procedures Medical Equipment Medical History Medications List Payer Information Physical Exam Problems List Review of Systems Social History Surgical/Procedural History Vital Signs Page 23 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD ToC CEDD Objects ToC CEDD Objects in Detail Within this section, ToC CEDD Objects are listed alphabetically and contain a summary and details at the ToC CEDD Data Element level. Each ToC CEDD Object summary includes a definition, CDA ID references, clinical applications, and logical relationships to other ToC CEDD Objects. ISO/HL7 Data Type: Please note that B and C Priority Objects may require more work to finalizing typing of included data elements. Further explanation on data types is provided in the Usage of HL7/ISO Data Types section. Examples and guidance are provided including clinically relevant terminology from CDA as well as potential vocabularies and value sets to use in storing these ToC CEDD Data Elements. Sections of the S&I Framework CEDD Value Set Index indicate ToC recommended value sets. Structure is listed as the data type of child objects. For certain data elements, examples and guidance information may be missing due to the following reasons: A clinical example may be deemed unnecessary due to direct inference from data element name Structure may be listed as a data type due to insufficient development at the time of this ToC CEDD version publication. A CDA reference may not be listed due to alignment with a CDA section rather than entry, an accurate equivalent may not exist in CDA at this time, or may not have been developed by the time of this ToC CEDD version publication Expected value sets may not listed for certain coded entries due to the controlled nature of applicable vocabularies For the aforementioned reasons, blank entry fields are denoted through light grey coloring to signify recognition of the known missing, insufficient, or unavailable information. Page 24 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Admitting and Discharging Diagnoses Definition Admitting Diagnoses are the diagnoses assigned to a patient at the time of admission to a facility. Discharge Diagnoses are the diagnoses assigned to a patient on discharge from a facility. These terms are consistent with admission to a facility and not applicable to the ambulatory environment. Admitting and Discharging Diagnoses Object Summary Clinical Application CDA ID Reference(s) Admitting Diagnoses: Diabetic Ketoacidosis, Type II Diabetes, Hyperlipidemia, Obesity, Noncompliance Discharge Diagnoses: Type II Diabetes, Hyperlipidemia, and Obesity. Admitting and discharge diagnosis 2.16.840.1.113883.10.20.22.2.24 might or might not be the same. Admitting diagnosis might often be prospective or might be a chief complaint that represents a health concern or symptom, e.g. chest pain. Admitting and Discharging Diagnoses Object in Detail Data Element ISO/HL7 Data Element Definition Clinical Example Name Data Type Hospital The diagnosis(es) that was the reason Admission for hospitalization at the time of Appendicitis CD Diagnosis hospitalization The diagnosis(es) determined to be Hospital the reason for hospitalization at the Myocardial infarction, Discharge time of discharge (this may be the CD 90% occlusion of the LAD Diagnosis same or different from the hospital admission diagnosis Prospect of recovery as anticipated Critical, Guarded, fair, Patient Conditions CS from the usual course of disease or stable, good Parent Objects Discharge Summary Encounters CDA Reference Child Objects Problems List Expected Value Set 1.14 Care Transition- Problem Value Set 1.14 Care Transition- Problem Value Set Page 25 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Data Element Name Problem Observations Reason for Admission Admitting and Discharging Diagnoses Object in Detail ISO/HL7 Data Element Definition Clinical Example Data Type peculiarities of the case All of the patients active Problems List Object Structure medical problems Narrative description of the primary Chest Pain ED reason for admission to a facility CDA Reference Expected Value Set Allergies and Intolerances Definition Captures a list of known allergies and intolerances, or no known allergies and intolerances. Allergic reactions occur when patients are exposed to an allergen an allergen can be a medication or an environmental compound (e.g. food, or pollen). Patients may also have adverse reactions to substances that are not true allergic reactions, known as intolerances. This list is comprised of the agents causing the allergic reaction or intolerance. Allergies and Intolerances Object Summary Clinical Application CDA ID Reference(s) Allergic reactions occur when patients are exposed to an allergen an allergen can be a medication or an environmental compound (e.g. food, or pollen). Patients may also have adverse reactions to substances that are not true allergic reactions, known as intolerances. A patient with an allergic reaction to shellfish may develop anaphylactic shock after ingesting shellfish. Parent Objects 2.16.840.1.113883.10.20.21.2.6.1 2.16.840.1.113883.10.20.21.2.6. Child Objects Consultation Request including Clinical Summary Consultation Summary Discharge Instructions Discharge Summary Page 26 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Data Element Name A/I Attributes Environmental Allergens Food Allergens List of Reactions Medication Intolerance Reaction Attributes Reaction Date Allergies and Intolerances Object in Detail ISO/HL7 Data Element Definition Clinical Example Data Type Veracity of the data based on source Older patient has been and details available about index told that had a Rx as a reaction(e.g. older patient has been child vs. clinician has told that had a Rx as a child vs. ED healthcare professional clinician has healthcare professional documentation of an documentation of an anaphylactic anaphylactic episode episode) Examples of A list of associated environment environmental allergens allergens for the medication includes CE include latex, pollen, seasonal allergens. animal dander, etc... A list of associated food allergens for the medication A list of reactions from allergies/intolerances Medication (ingredient or class code, if available) that has been attributed to an allergic reaction or intolerance, or drug code if attribution to ingredient or class is unavailable Clinical statement detailing an undesired symptom, finding, etc., due to an administered or exposed substance. Date when this particular Intolerance Condition or Allergy first manifested Examples of food allergens include shellfish, eggs, peanuts, etc. (e.g. anaphylaxis), nausea, morbilliform skin rash e.g. Opiates Includes medications, biologicals, herbal supplements, OTCs, vaccine, etc. Acute generalized peritonitis (disorder) CDA Reference Product Coded CE Product Coded LIST Reaction FreeText ED Product FreeText CE Reaction Coded Expected Value Set 1.3 Allergy/Adverse Event Food and Other Allergens Value Set 1.3 Allergy/Adverse Event Food and Other Allergens Value Set 1.4 Allergy/Adverse Event Reaction Value Set TS Page 27 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Reaction Identified By Allergies and Intolerances Object in Detail ISO/HL7 Data Element Definition Clinical Example Data Type itself or was confirmed via testing if it had not yet manifested itself. e.g. patient, provider, care Who reported the reaction EN taker Reaction Type Describes type of reaction Data Element Name Severity Attributes Clinical statement detailing severity of a specific reaction Severity of Intolerance or Allergy Severity associated with the reaction. This is a description of the level of severity of the allergy or intolerance Propensity to adverse reactions (disorder) Mild to moderate (qualifier value) A patient was treated in the ED and hospitalized overnight 3 years ago for severe anaphylaxis 30 minutes after eating roasted peanuts; six months ago they ate a dish served with a utensil that had been contaminated with peanut sauce and had itching of their mouth that resolved after Benadryl; their condition is considered a severe peanut allergy, even though they have had a mild episode on one occasion CDA Reference CE CD Severity Coded ED Severity FreeText Expected Value Set 1.5 Allergy/Adverse Event Type Value Set 1.19 Care TransitionSeverity Value Set Page 28 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Behavioral Health History Definition Specifies the summary report intended to exchange selected information relevant across specialties. It may not include the details of an assessment but it will contain many data elements that are based on the information collected through the assessment and generated from its processing. May often include information that would be considered sensitive information. Data Element Name Confidentiality Code DSM Axis 1 Behavioral Health Object Summary Clinical Application CDA ID Reference(s) Parent Objects Child Objects History of conditions or episodes that would fall in the behavioral health domain, such as a history of depression treated by the patients previous PCP with antidepressant medications and an inpatient stay in a behavioral health facility. Behavioral Health History Object in Detail ISO/HL7 Data Element Definition Clinical Example Data Type This attribute is used to specify that the content of this clinical document is INTEGER sensitive because it contains Behavioral Health information The DSM-IV organizes each psychiatric diagnosis into five dimensions (axes) Structure relating to different aspects of CDA Reference Expected Value Set Page 29 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Data Element Name Behavioral Health History Object in Detail ISO/HL7 Data Element Definition Clinical Example Data Type disorder or disability: CDA Reference Expected Value Set Axis I: Clinical disorders, including major mental disorders, and learning disorders The DSM-IV organizes each psychiatric diagnosis into five dimensions (axes) relating to different aspects of disorder or disability: DSM Axis 2 DSM Axis 3 DSM Axis 4 Axis II: Personality disorders and intellectual disabilities (although developmental disorders, such as Autism, were coded on Axis II in the previous edition, these disorders are now included on Axis I) The DSM-IV organizes each psychiatric diagnosis into five dimensions (axes) relating to different aspects of disorder or disability: Axis III: Acute medical conditions and physical disorders The DSM-IV organizes each psychiatric diagnosis into five dimensions (axes) relating to different aspects of disorder or disability: Structure Structure Structure Page 30 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Data Element Name DSM Axis 5 Environmental Factors GAF Score Behavioral Health History Object in Detail ISO/HL7 Data Element Definition Clinical Example Data Type Axis IV: Psychosocial and environmental factors contributing to the disorder The DSM-IV organizes each psychiatric diagnosis into five dimensions (axes) relating to different aspects of disorder or disability: Structure Axis V: Global Assessment of Functioning or Children's Global Assessment Scale for children and teens under the age of 18 Description of environmental factors Structure affecting patient Global Assessment of Functioning (GAF) Range from 0-100, e.g. 50 INT CDA Reference Expected Value Set Part of the diagnosis on Axis 5 Homicidal Ideation Clinical statement describing patient thoughts about homicide Suicidal Ideation Clinical statement describing patient thoughts about suicide Treatment Referral Description of specialized treatment referrals Patient reports fantasizing about killing his spouse with his gun. Patient reports thinking about jumping out of a window of a high story building HIST HIST Structure Page 31 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Care Team Members Definition A list of the care team members and their role in the patient’s care. Care Team Members Object Summary Clinical Application CDA ID Reference(s) In an advanced primary care model the care team would include anyone actively involved in the patient's care such as the PCMH team, the patient’s designees, entities providing care and all additional caregivers designated by the PCP or designated provider. Parent Objects Child Objects Encounters Primary Care and Designated Providers Care Team Members Object in Detail Data Element Name Care Team Provider Care Team Roles Care Team Member ID Data Element Definition Provider information from Primary Care Physicians and Designated Providers The role on the care team Includes non-physician providers such as physical therapist, LCSW, nutritionist, who might be part of care team. Provider Index number Clinical Example ISO/HL7 Data Type CDA Reference Expected Value Set Structure PCP, embedded care manager, social worker, specialist consulting physician, etc. ED Unique identifier, such as NPI for providers II Provider Role Free Text Page 32 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Consult(s) Assessments and Plan(s) Recommendations Consult(s) Assessments and Plan(s) Object Summary Clinical Application CDA ID Reference(s) Definition Contains information as part of a patient assessment that was performed, plan of care recommendations, and/or consultation reasons Data Element Name Assessment Narrative Assessment or Recommendation Date Author List of Associated Medications Plan of Care Goals These data elements include any non-core assessments, plans, and orders, including free text of the consultant's assessments and plan recommendations. 2.16.840.1.113883.10.20.22.1.9 Parent Objects Consultation Request including Clinical Summary Consultation Summary Discharge Instructions Discharge Summary Consult(s) Assessments and Plan(s) Recommendations Object in Detail ISO/HL7 CDA Reference Data Element Definition Clinical Example Data Type The clinician's conclusions and working assumptions that will guide ST treatment of the patient. Date of assessment or care plan establishment TS Specific clinician author of care plan or assessment PN Medications List Object Structure Goals Object Structure Child Objects Goals Invasive and Non-Invasive Procedures Medications List Reason for Consult Request Expected Value Set Page 33 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Data Element Name Plan of Care Procedure Provisional Diagnosis Request Reason Status of Recommendation Consult(s) Assessments and Plan(s) Recommendations Object in Detail ISO/HL7 CDA Reference Data Element Definition Clinical Example Data Type Invasive and Non-Invasive Procedures Structure Object Description of unconfirmed diagnosis to be addressed through assessment CD or plan Reason for Consult Request Object Structure Describes state of assessment or plan Proposal, request, etc. CS recommendation Expected Value Set 1.14 Care Transition- Problem Value Set Culturally Sensitive Patient Care Definition Information specific to the patient's cultural, religious, and educational background. Data Element Name Confidentiality Code Disability Educational Level Culturally Sensitive Patient Care Object Summary Clinical Application CDA ID Reference(s) Patient’s thyroid nodule FNA demonstrated follicular thyroid 2.16.840.1.113883.10.20.21.1.1 cancer; patient has been scheduled [US Realm Document Header] for surgery in one month. Culturally Sensitive Patient Care Object in Detail ISO/HL7 Data Element Definition Clinical Example Data Type This field contains information about e.g. highly sensitive, not the level of security and/or sensitivity CE sensitive, sensitive surrounding the order The disability status of the patient Deaf CE Acceptable values for this data Graduate Degree CE element include the following Parent Objects Child Objects Demographics CDA Reference Expected Value Set Page 34 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Data Element Name Ethnicity Language Race Religion Culturally Sensitive Patient Care Object in Detail ISO/HL7 Data Element Definition Clinical Example Data Type (Advanced Degree, College Graduate, Some College, High School Graduate, Elementary) Ethnicity is a term that extends the Latino CE concept of race. The coding of ethnicity is aligned with public health and other federal reporting standards of the CDC and the Census Bureau Language will be identified as spoken, Arabic CE written, or understood; but no attempt will be made to assess proficiency. The default language is English, but English is to be entered explicitly similar to any other listed language Race is usually a single valued term that may be constant over that patient's lifetime. The coding of race is aligned with public health and other federal reporting standards of the Asian CE CDC and the Census Bureau. Typically the patient is the source of the content of this element. However, the individual may opt to omit race. Religious affiliation of the patient Catholic CE CDA Reference Expected Value Set Ethnicity Language Race Religious Affiliation Page 35 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Demographics Demographics Object Summary Clinical Application CDA ID Reference(s) Definition Parent Objects The Demographics CEDD Object would assemble multiple child objects into a Demographics parent object Captures relevant patient information at an instance of care 2.16.840.1.113883.10.20.21.1.1 [US Realm Document Header] Consultation Request including Clinical Summary Consultation Summary Discharge Instructions Discharge Summary Child Objects Culturally Sensitive Patient Care Existence of Advance Directives Patient Contact Information Patient Information Payer Information Demographics Object in Detail Data Element Name ID Patient Advance Directives Patient Contact Information Patient Cultural Sensitive Information Data Element Definition Clinical Example ISO/HL7 Data Type A unique identifier for the Demographics CEDD Object II Existence of Advance Directives Object Structure Patient Contact Information Object Structure Culturally Sensitive Patient Care Object Structure CDA Reference Expected Value Set Page 36 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Demographics Object in Detail Data Element Name Patient Information Patient Payer Information Data Element Definition Patient Providers Patient Support Contacts Clinical Example ISO/HL7 Data Type Patient Information Object Structure Payer Information Object Structure Primary Care and Designated Providers Object Structure Support Contacts Object Structure CDA Reference Expected Value Set Diet and Nutrition Definition Information specific to the patient's cultural, religious, and educational background. Diet and Nutrition Object Summary Clinical Application CDA ID Reference(s) Patient’s thyroid nodule FNA demonstrated follicular thyroid 2.16.840.1.113883.10.20.21.1.1 cancer; patient has been scheduled [US Realm Document Header] for surgery in one month. Parent Objects Child Objects Discharge Summary Diet and Nutrition Object in Detail Data Element Name Diet Description Data Element Definition Narrative of the recommended diet or daily nutrient intake Clinical Example ISO/HL7 Data Type CDA Reference Expected Value Set General Healthful Diet; 80 gm protein + Consistent Carbohydrate + ED 2g sodium + 2g potassium + 800-1000mg Phosphorus + 1500 mL Fluid Restricted Page 37 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Diet and Nutrition Object in Detail Data Element Name Diet Type Code Discharge Diet Food Type Code Nutrition Assessment Nutrition Care Provider Nutrient Modification Required ISO/HL7 Data Type Data Element Definition Clinical Example Set of codes that controls the type of diet modification that a patient should receive or follow Records a narrative description of the expectations for diet, including proposals, goals, and order requests for monitoring, tracking, or improving the dietary control of the patient, used in a discharge from a facility such as an emergency department, hospital, or nursing home. DASH (Dietary Approaches to Stop Hypertension), Kosher, or Vegan LIST Low-fat, low-salt, cardiac diet ED Patient undergoing treatment and rehabilitation following a stroke may require honeythickened liquids, ground meats and chopped vegetables CE BMI:22 PQ Indicates what type of food, such as meats, or liquids, which require a texture modification Anthropometric measurement outcomes Nutrition professional (RD) responsible for completing the request nutrition consult and developing the nutrition prescription, and nutrition plan of care Indicator specifying whether the patient/client requires a therapeutic or modified diet to eliminate, decrease, or CDA Reference Expected Value Set EN Diabetic patient requires controlled intake of carbohydrates. BL Page 38 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Diet and Nutrition Object in Detail Data Element Name Data Element Definition Clinical Example ISO/HL7 Data Type CDA Reference Expected Value Set increase certain substances in the diet (e.g., sodium, potassium) Nutrition Monitoring and Evaluation Nutrient Type Code Narrative includes both patient evaluation of goals, re-assessment of existing parameters and evaluation Patient has reduced sodium and cholesterol consumption and reduced BMI from 30 to 27. Outcomes over past 9 months reflect a reduction in BP and LDL . ED Code which identifies the nutrient which is to be modified Sodium or Protein CE Nutrition Observation Food and nutrition related observation narrative of indicators which are used to evaluate the nutritional status of the patient. Nutrition Diagnosis Identification and labeling of a nutrition problem that a food and nutrition professional is responsible for treating independently Nutrition Intervention Purposefully planned actions intended to positively change a nutrition-related behavior, environmental condition, or aspect of health status for an individual Three-day food record reflects patient has been severely restricted in protein/kcalorie intake. Average intake was 1250 kcal, 30 grams protein. Biting/Chewing (masticatory) difficulty (SNOMED CT CID 175130015 ) related to xerostomia as evidenced by Speech Language Pathologist evaluation. Adaptive equipment for feeding assistance ED CE ED Page 39 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Diet and Nutrition Object in Detail Data Element Name Data Element Definition Clinical Example Nutrition Prescription The patient’s individualized recommended dietary intake of energy and/or selected foods or nutrients based on current reference standards and dietary guidelines and the patient’s health condition and nutrition diagnosis. Recommend patient consume 2000 calories, 80 grams protein per day for optimal wound healing. ED Biochemical Data, Medical Tests and Procedures and tests Lab data: electrolytes, glucose; Tests: gastric emptying time, resting metabolic rate PQ Nutrition Results PES Statement Quantity of Nutrient Describes Problem/Etiology Signs/Symptoms Indicates how much of the nutrient is being ordered or recommended ISO/HL7 Data Type CDA Reference Expected Value Set ED PQ Encounters Definition Captures the details of a specific patient encounter event. Encounters Object Summary Clinical Application CDA ID Reference(s) Details information on the encounter event and captures the patient health information relevant to the encounter event. 2.16.840.1.113883.10.20.22.2.22 Parent Objects Consultation Request including Clinical Summary Consultation Summary Discharge Child Objects Care Team Members Problems List Page 40 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Definition Encounters Object Summary Clinical Application CDA ID Reference(s) Parent Objects Instructions Discharge Summary Child Objects Encounters Object in Detail Data Element Name Encounter Attributes Encounter Care Team Encounter Date/Time Encounter ID Encounter Problem List Encounter Provider Encounter Type Patient Class ISO/HL7 Data Type CDA Reference Narrative describing the encounter; ED Encounter Free Text Care Team Member Object Structure Data Element Definition Clinical Example Date and time of the encounter May include duration if pertinent An identifier for the encounter event TS II Expected Value Set Encounter Date/Time Encounter ID Problems List Object Structure Name and other information for the person or organization that performed or hosted the encounter EN Encounter Provider Describes the type of encounter Out Patient Office Visit CE Encounter Type Categorizes patients by the site where the encounter occurred. Emergency, Inpatient CE Patient Class 1.21 Encounter Type Value Set 1.12 Care TransitionPatient Class Value Set Page 41 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Existence of Advance Directives Existence of Advance Directives Object Summary Clinical Application CDA ID Reference(s) Definition Captures the existence of advanced directives for a patient; simply whether or not the patient had advanced directives is relevant, but type is also able to be specified Data Element Name Advanced Directives Existence Advance Directive Owner Advance Directive Range Advance Directive Type Patient has discussed advanced directives with one of their treating clinicians, made decisions about their wishes and completed an AD form. 2.16.840.1.113883.10.20.22.2.21 Existence of Advance Directives Object in Detail ISO/HL7 Data Element Definition Clinical Example Data Type Describes the type of the advance directive Name, address, or other contact information of the person for the person or organization that can provide a copy of the document The effective date of the advance directive Coded value indicating the type of advance directive Life Support Parent Objects Consultation Request including Clinical Summary Consultation Summary Discharge Instructions Discharge Summary Demographics CDA Reference ED Advance Directive Free Text Type EN Custodian of the Document TS Effective Date CE Advance Directive Type Child Objects Expected Value Set 1.2 Advance Directive Type Value Page 42 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Existence of Advance Directives Object in Detail ISO/HL7 Data Element Definition Clinical Example Data Type Data Element Name CDA Reference Expected Value Set Set Family History Definition The patient's family history data elements which are not a summary, as the sending physician may want to select specific elements for inclusion. Family History Object Summary Clinical Application CDA ID Reference(s) Patient has a family history significant for: mother died of colon cancer at age 48, maternal grandmother, paternal 2.16.840.1.113883.10.20.22.2.15 grandfather, and father with hypertension; maternal grandfather with unknown cancer, deceased age 52. Parent Objects Child Objects Consultation Request including Clinical Summary Consultation Summary Family History Object in Detail Data Element Name Family History Genetic Relative Administrative Gender Data Element Definition Textual description about the problems, diagnoses, and genetic markers found in genetic relatives. This field may be used to capture unstructured or structured family history information recorded in clinical records. gender (i.e., the behavioral, cultural, or psychological traits typically associated with one sex) of the genetic relative as ISO/HL7 Data Type CDA Reference Maternal Grandmother with history of Alzheimer's Dementia; Paternal Grandfather with CAD and HTN, deceased MI age 58 HIST Family Member Information Female CE Family Member Administrative Gender Clinical Example Expected Value Set Page 43 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Family History Object in Detail Data Element Name Data Element Definition Clinical Example ISO/HL7 Data Type Patient has had individual genome analysis that revealed BRACA 1 Structure CDA Reference Expected Value Set defined for administrative purposes Genetic Marker Description Description of risk-related genetic markers identified in this individual. Genetice Relative Age at Death Represents the subject's age at onset of an event or observation Genetic Relative Cause of Death Observation Indicates that a particular problem was the cause of death of the family member Genetic Relative Condition Condition is the generic term used in the model to designate conditions, problems, diagnoses, etc. Genetic Relative Date of Birth Date of birth of the genetic relative Genetic Relative Ethnicity The cultural heritage with which the genetic relative identifies themselves PQ Family Member Age (at death) A common scenario is that a patient will know the age of a relative when the relative had a certain condition or when the relative died, but will not know the actual year (e.g., "grandpa died of a heart attack at the age of 50"). Often times, neither precise dates nor ages are known (e.g. "cousin died of congenital heart disease as an infant"). CD Family Member Cause of Death "cousin has systemic lupus erythematosus" CE Family Member Condition TS Latino CE Family Member Date of Birth Family Member Ethnicity Page 44 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Family History Object in Detail Data Element Name Data Element Definition Clinical Example ISO/HL7 Data Type Details about problems or diagnoses for this genetic relative. Specifies if the family member is a twin, triplet etc. and whether identical or fraternal Clinical Example: Type II Diabetes Structure Patient is second of a twin birth CF Genetic Relative Problem Status Status of the genetic relative’s problem Active, inactive CS Genetic Relative Name Name of family member. For privacy reasons this may not be appropriate for sharing or public display and in this situation the 'label' should be used. John Doe PN Genetic Relative Race Race of the genetic relative Asian CE Genetic Relative Relationship The relationship of the genetic relative to the individual. Clinical Example: Mother CE Genetic Relative Medical History Genetic Relative Multiple Birth Status CDA Reference Expected Value Set Family Member Medical History Family Member Multiple Birth Status Family Member Problem Status Family Member Name Family Member Race Family Member Relationship General Results Definition The patient's family history data elements which are not a summary, as the sending physician may want to select specific elements for inclusion. General Results Object Summary Clinical Application CDA ID Reference(s) Patient has a family history significant for: mother died of colon cancer at age 48, maternal grandmother, paternal 2.16.840.1.113883.10.20.22.2.15 grandfather, and father with hypertension; maternal grandfather with unknown cancer, deceased age 52. Parent Objects Consultation Request including Clinical Summary Consultation Summary Discharge Child Objects Page 45 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD General Results Object Summary Clinical Application CDA ID Reference(s) Definition Parent Objects Summary Child Objects General Results Object in Detail Data Element Name Data Element Definition Result Type Coded representation of the observation performed Result Narrative Description of type of results Date Result Obtained Date and time of the results Result Status Status of this observation Result Interpretation Result Value An abbreviated interpretation of the observation Value of the result Result Reference Range Reference range for the observation Clinical Example Hematology, Chemistry, Nuclear Medicine microscopic examination of the tissue received marked "lung biosy" shows adenocarcinoma ISO/HL7 Data Type CDA Reference Expected Value Set CD Result Type 1.40 Results Value Set ED TS Result Date/Time Active, aborted CS Result Status Normal, high CE 13.2 g/dl PQ M 13-18 g/dl; F 12-16 g/dl 1.39 Result Status Value Set Result Interpretation Result Value Result Reference Range Goals Definition This is a list of the healthrelated goals, such as Goals Object Summary Clinical Application CDA ID Reference(s) The patient and the clinician have discussed and agree on the Parent Objects Consult(s) Assessment(s) Child Objects Page 46 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Definition smoking cessation agreed upon by the patient and the physician. Goals Object Summary Clinical Application CDA ID Reference(s) patient’s goal of 5 lbs. of weight loss over the next 2 months. Goals might or might not have a time frame. For example, maintaining an HgbA1 below a certain level might be a goal for a diabetic. Parent Objects and Plan(s) Recommendations Child Objects Goals Object in Detail Data Element Name Author Data Element Definition The person who records the goal. What actually happens. Quantifiable Actual Outcome measureable finding, observation or result What is expected to happen. Desired Outcome Quantifiable measurable description added to Goal description. Goal agreed to by Any person who agrees to supporting the goal Goal Category The goal type Goal Description Goal Established date/time The human readable text describing what is expected to happen Date and time goal is entered/identified Clinical Example ISO/HL7 Data Type Nurse, PT, patient, MD. The MD records goal for patient to ambulate. PN Patient Ambulated 15 feet. ED Ambulate 20 feet. Structure Nurse, PT, patient, MD, Patient agrees to goal of walking 20 feet. Activity, Diet, medication, learning etc. Patient will ambulate CDA Reference Expected Value Set PN ST ST TS Page 47 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Goals Object in Detail Data Element Name Data Element Definition Clinical Example Goal Intent The overarching outcome targeted Patient will return to baseline status of full free unlimited ambulation state as prior to admission Goal Priority The ranking of the goal compared to other goals 1, 2, 3, etc… Goal Reviewed by Goal Revised By Goal Status Goal Target Date/Time Any person who reviews the goal Any person who edits or refines the goal The particular stage within the defined goal process (based on QDM/NQF status definition draft October 2011) The date and time when the measurement should be taken, goal should be reached. Nurse reviews goal of patient’s ambulation goal as set and refined by MD and PT PT refines goal to patient will ambulate 20 feet ISO/HL7 Data Type ED INT CDA Reference Expected Value Set PN PN The goal is Met, not met, in progress, or on hold. CS May be specific end date, or may have a range date (beginning and end) IVL_TS History of Present Illness Definition In a medical encounter, a history of the present illness (abbreviated HPI) [1] (termed history of presenting complaint (HPC) in the UK) refers to a History of Present Illness Object Summary Clinical Application CDA ID Reference(s) Patient reports having new onset chest pain described as a dull pain like an elephant sitting on his chest. 1.3.6.1.4.1.19376.1.5.3.1.3.4 Pain radiates down the arm, is relieved with rest, began 1 week ago, and lasts for minutes. Pain is Parent Objects Consultation Request including Clinical Summary Consultation Child Objects Page 48 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Definition detailed interview prompted by the chief complaint or presenting symptom (for example, pain). Data Element Name History of Present Illness History of Present Illness Object Summary Clinical Application CDA ID Reference(s) brought on with stress or climbing stairs. History of Present Illness Object in Detail ISO/HL7 Data Element Definition Clinical Example Data Type Patient presents with a 24 hour history of urinary frequency, urgency, and painful urination. She Historical details leading up to and reports that she had pertaining to the patient’s current sexual intercourse with a HIST complaint or reason for seeking new male partner ~ 48 medical care hours ago and denies fevers, abdominal, or flank pain, urinary or vaginal bleeding Parent Objects Summary Discharge Summary CDA Reference Child Objects Expected Value Set Immunization History Definition A list of the immunizations that the patient has received including date of immunization, where the Immunization History Object Summary Clinical Application CDA ID Reference(s) The patient’s immunization history includes BCG, or bacille Calmette2.16.840.1.113883.10.20.22.2.2.1 Guérin which is a vaccine for TB, as 2.16.840.1.113883.10.20.22.2.2 an infant. Parent Objects Child Objects Consultation Request including Page 49 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Definition immunization was administered if known, and lot or batch number if available. Immunization History Object Summary Clinical Application CDA ID Reference(s) Parent Objects Clinical Summary Consultation Summary Discharge Instructions Discharge Summary Child Objects Immunization History Object in Detail Data Element Name Category of Immunization Data Element Definition Clinical Example ISO/HL7 Data Type CDA Reference Expected Value Set CE Coded Product Name 1.25 Immunizations Administered Vaccine Value Set TS Administered Date ST Lot Number What the immunization is for Potential for exposure to pertussis ED Contraindication Reason to not give the immunization in the future due to previous reaction or other existing condition Allergy to component or immunodeficiency state BL Immunization Administered Coded immunization description from a controlled vocabulary Immunization Date Immunization Lot Number Immunization Manufacturer Name Immunization The date and time the immunization was administered The manufacturer’s production lot number for the administered product Cholera Manufacturer of immunization Sanofi Pasteur ED Free Text Product Name Indicates which type of series the Current valid values are IVL_INT Medication Page 50 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Immunization History Object in Detail Data Element Name Series Number Immunization Facility Immunization Performer Immunization Route Observed Reaction Site of Delivery Refusal Reason Data Element Definition patient has been given. Clinical Example ISO/HL7 Data Type Series 1 through 8, Partially complete, booster, or complete EN Person who administered the immunization PN The response of cells or tissues to an antigen, as in a test for immunization Body site where immunization was administered Documents the rationale for the patient declining an immunization Intranasal, subcutaneous, intradermal The observed response to an antigen which would normally be a description of skin reaction including size and time since test was applied, as in a test for immunization to be given or for tuberculosis Expected Value Set Series Number Facility performing immunization How immunization is administered CDA Reference Performer 1.30 Medication Route Value Set CE COLL Reaction Left deltoid arm CE Site "Vaccine safety concerns" CE Refusal Reason 1.4 Allergy/Adverse Reaction Value Set 1.6 Care TransitionBody Site Value Set 1.26 Immunization Reason Value Set Invasive & Non-Invasive Procedures Definition A listing of all invasive and non-invasive procedures for a patient. Invasive and Non-Invasive Procedures Object Summary Clinical Application CDA ID Reference(s) Defines all interventional, surgical, diagnostic, or therapeutic 2.16.840.1.113883.10.20.22.2.7.1 procedures or treatments pertinent Parent Objects Child Objects Consultation Request Page 51 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Definition Data Element Name Entity Performing Procedure Entity Performing Procedure Address Entity Performing Procedure Phone Number Invasive and Non-Invasive Procedures Object Summary Clinical Application CDA ID Reference(s) to the patient historically at the time the document is generated. May contain all procedures for the period of time being summarized, but should include notable procedures. Invasive and Non-Invasive Procedures Object in Detail ISO/HL7 Data Element Definition Clinical Example Data Type Identifies the location where the procedure was performed Critical Care Unit AD Phone number of entity performing the procedure TN Invasiveness of Procedure Describes invasiveness of the procedure Procedure Records clinically significant CDA Reference Child Objects Expected Value Set EN Address of entity performing the procedure Non-invasive (ex: abdominal sonogram), minimally invasive (ex: endoscopy), invasive (open surgery) Necrotic left ovarian cyst Parent Objects including Clinical Summary Consultation Summary Discharge Summary CS ED Page 52 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Data Element Name Findings Procedure Implants Procedure Narrative Procedure Performed Procedure Provider Procedure Specimens Taken Procedure Time Site of Procedure Invasive and Non-Invasive Procedures Object in Detail ISO/HL7 Data Element Definition Clinical Example Data Type observations confirmed or discovered was discovered and during the procedure or surgery. removed Records any materials placed during A doohickey pacemaker, the procedure including stents, tubes, number 3975359 was ED and drains. placed A 6 mm sessile polyp was found in the ascending colon and removed by Narrative to further describe the snare, no cautery. ED procedure Bleeding was controlled. Moderate diverticulosis and hemorrhoids were incidentally noted. This code could come Contains a code indicating a procedure from various coding or a non-procedural event involving the systems; typically the CD patient Common Procedure Terminology (CPT) Provider performing procedure Records the tissues, objects, or samples taken from the patient during the procedure including biopsies, aspiration fluid, or other samples sent for pathological analysis Date and time of procedure, may include duration Anatomical site where procedure is PN a .01x .02 cm biopsy was taken from the left ventricle Expected Value Set Procedure Free Text Type Procedure Type 1.34 Procedure Value Set Procedure Provider ED IVL_TS Skin biopsy of the left CDA Reference CD Procedure Date/Time Body Site 1.6 Care TransitionPage 53 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Data Element Name Times Performed Invasive and Non-Invasive Procedures Object in Detail ISO/HL7 Data Element Definition Clinical Example Data Type performed eyelid Indicates the number of times this procedure was performed for the patient at one setting During the laparoscopic Most procedures are only performed thermal liver tumor (and recorded) once; this property INT ablation 3 different areas allows for the recording of multiple of tumor were ablated procedures in order to remove the necessity to record the same procedure multiple times. CDA Reference Expected Value Set Body Site Value Set Medical Equipment Definition Medical Equipment Object Summary Clinical Application CDA ID Reference(s) Durable Medical Equipment Crutches, neck brace or cane (DME), and any other ordered for the patient equipment ordered for the patient. 2.16.840.1.113883.10.20.22.2.23 Parent Objects Consultation Request including Clinical Summary Consultation Summary Discharge Instructions Discharge Summary Child Objects Page 54 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Medical Equipment Object in Detail Data Element Name Equipment Code Equipment Date Acquired Equipment Date Disposed Equipment ID Equipment Model Name Equipment Owner Equipment Software Name Equipment Software Version Equipment Status Quantity of Equipment Data Element Definition Clinical Example Coded manufacturer name Date and time equipment was installed or affixed Date and time equipment was removed or uninstalled Unique identifier for the device The human designated moniker for a device, assigned by the manufacturer Entity or person who owns the device CDA Reference Expected Value Set Playing Device DATE II CK EN The moniker, version and release of the software that operates the device as assigned by the software manufacturer or developer Version of the equipment software Describes state or condition of equipment Number of devices identified by the Equipment ID ISO/HL7 Data Type CE DATE CK INT Activated; New CS PQ Quantity Medical History Definition The patient's previous medical problems. Medical History Object Summary Clinical Application CDA ID Reference(s) Patient with a past medical history of gallstones x 2 episodes which 2.16.840.1.113883.10.20.22.2.20 resolved post cholecystectomy Parent Objects Consultation Request including Child Objects Page 55 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Medical History Object Summary Clinical Application CDA ID Reference(s) Definition Parent Objects Clinical Summary Consultation Summary Discharge Summary Child Objects Medical History Object in Detail Data Element Name Medical History Data Element Definition Describes all aspects of the medical history of the patient even if not pertinent to the current encounter The history may be limited to information pertinent to the current procedure or may be more comprehensive. The history may be reported as a collection of random clinical statements or it may be reported categorically. Clinical Example Patient has a history of asthma with 3 episodes of hospitalization, 2 of which resulted in intubation, both for less than 72 hours. ISO/HL7 Data Type CDA Reference Expected Value Set LIST Medications List Definition A list of medications that patient should be taking or an entry of no known medications. The list of Medications List Object Summary Clinical Application CDA ID Reference(s) The list of all of the medications 2.16.840.1.113883.10.20.22.2.1.1 that the patient is taking, or has 2.16.840.1.113883.10.20.22.2.1 been prescribed, and the patient is 2.16.840.1.113883.10.20.22.2.38 thought to be taking. If a clinician Parent Objects Consultation Request including Clinical Child Objects Page 56 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Definition medications includes compounds that the patient may be taking (e.g. herbals). The metadata for the Medication List is to include: the clinician that last ordered the medication with the date/time stamp of when the medication was last ordered, and whether or not the Medication List was reconciled during this encounter and if so by whom, and if not when last reconciled and by whom. Medications List Object Summary Clinical Application CDA ID Reference(s) reads the patient a list of their medications and the patient reports that they actually stopped taking medication “X”, medication X would be removed from the list. D/C reconciliation would include consideration of the prehospitalization medications and whether these need to be continued or stopped. Parent Objects Summary Consultation Summary Discharge Instructions Discharge Summary Child Objects Medications List Object in Detail Data Element Name Data Element Definition Active Medications A list of clinically relevant medications. Includes: PRN Medication List, Active Medications (Held for Period of Time), Medications that patient was exposed to, now discontinued, but still clinically relevant, Software need – document the delta Clinical Example Lipitor 20 mg ISO/HL7 Data Type CDA Reference Expected Value Set CE Coded Product Name, Coded Brand Name, Free Text Product Name, Free Text Brand Name 1.9 Care TransitionMedication Brand Name 1.10 Care TransitionsMedication Clinical Drug Name Page 57 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Medications List Object in Detail Data Element Name Data Element Definition Clinical Example ISO/HL7 Data Type CDA Reference Includes ICD-9 codes and/or SNOMED codes Associated Assessment Reason the provider prescribed the medication Changed Medications Medications that have been modified in this encounter, i.e. dosage adjustments Date Of Reconciliation The date of the last active medication list reconciliation Discontinued Medications Medications that have been discontinued Expected Value Set 1.11 Care TransitionMedication Drug Class Hyperlipidemia: Lipitor Lipitor 10 mg discontinued; Lipitor 20 mg prescribed BAG CE Indication Coded Product Name, Coded Brand Name, Free Text Product Name, Free Text Brand Name 1.9 Care TransitionMedication Brand Name 1.10 Care TransitionsMedication Clinical Drug Name 1.11 Care TransitionMedication Drug Class Coded Product Name, Coded Brand Name, Free Text Product Name, Free Text Brand Name 1.9 Care TransitionMedication Brand Name 1.10 Care TransitionsMedication Clinical Drug Name 1.11 Care TransitionMedication Drug Class Value Set: Medication Brand DATE Lipitor 10 mg discontinued CE Page 58 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Medications List Object in Detail Data Element Name Data Element Definition Clinical Example ISO/HL7 Data Type CDA Reference Expected Value Set Name Dose Duration of Administration Frequency of Administration The amount of the product to be given. This includes a dose in measurable units (e.g., milliliters, or mg), the form (or administrative unit (e.g. tablets, suppository, etc...), and the amount of the form to take. For example Medication XXX 500 mg, tablets; take ½ tablet, administration 500 mg tablet unit (e.g., tablet), or an amount of active ingredient (e.g., 250 mg). May define a variable dose, dose range or dose options based upon identified criteria (see Dose Indicator) Need to have both the "dose" as well as the form or administration unit. The period of time that you are to take the medication if it is time For 10 days limited, e.g. take abx for 10 days Defines how often the medication is to be administered as events per unit of time. Often expressed as the number of times per day (e.g., four times a day), but may also include event6 hours while awake related information (e.g., 1 hour before meals, in the morning, at bedtime). Complimentary to Interval, although equivalent expressions may PQ Dose IVL Duration IVL_TS Frequency 1.9 Care TransitionMedication Brand Name Page 59 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Medications List Object in Detail Data Element Name Data Element Definition ISO/HL7 Data Type CDA Reference CE Product Form Prescription, over the counter drug CE Type of Medication Store in the refrigerator. Take with food. ED Patient Instructions PN Ordering Provider PN Provider Clinical Example Expected Value Set have different implications (e.g., every 8 hours versus 3 times a day) Medication Attributes Medication Type Patient Instructions Prescriber Reconciled By Route of Administration This is the physical form of the Tablet, capsule, liquid or product as presented to the individual. ointment Classification based on how the medication is marketed Instructions to the patient that are not traditionally part of the Sig. For example, “keep in the refrigerator.” More extensive patient education materials can also be included The person that wrote this order/prescription (may include both a name and an identifier) The name of the individual who last reconciled the active medication list Indicates how the medication is received by the patient (e.g., by mouth, intravenously, topically, etc.) By mouth; or apply to skin in area of rash CE Route Amputation stump CE Site Start Date Used to express the start date for a medication TS Indicate Medication Started Status of Indicate if the active medication list CS Site of Delivery The anatomic site where the medication is administered. Usually applicable to injected or topical products 1.29 Medication Product Form Value Set 1.31 Medication Type Value Set 1.30 Medication Route Value Set Page 60 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Medications List Object in Detail Data Element Name Reconciliation Stop Date Vehicle for Delivery When to Take Data Element Definition has been reconciled Used to express a "hard stop," such as the last Sig sequence in a tapering dose, where the last sequence is 'then D/C' or where the therapy/drug is used to treat a condition and that treatment is for a fixed duration with a hard stop, such as antibiotic treatment, etc. Non-active ingredient(s), or substances not of therapeutic interest, in which the active ingredients are dispersed. Most often applied to liquid products where the major fluid component is considered the vehicle. For PRN meds this information would be take when you are experiencing the system, e.g. take you nitroglycerine when you are experiencing chest pain ISO/HL7 Data Type CDA Reference TS Indicate Medication Stopped Normal Saline is the vehicle in “Ampicillin 150mg in 50ml NS”; Aquaphor is the vehicle in “10% LCD in Aquaphor” CE Vehicle At bedtime daily IVL_TS Administration Timing Clinical Example Expected Value Set 1.28 Medication Method for Delivery Value Set Operative Summary Definition Operative report containing details on operation performed and diagnoses pre and post operation. Operative Summary Object Summary Clinical Application CDA ID Reference(s) Operative Report Parent Objects Child Objects 2.16.840.1.113883.10.20.22.1.7 Page 61 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Operative Summary Object in Detail Data Element Name Postoperative Diagnosis Preoperative Diagnosis Operative Procedure Operative Summary Narrative Data Element Definition Records the diagnosis or diagnoses discovered or confirmed through the surgery Records the surgical diagnosis or diagnoses assigned to the patient before the surgical procedure and is to be confirmed through the surgery The operative procedure that was performed Narrative of the operation(s) performed Clinical Example ISO/HL7 Data Type CDA Reference Expected Value Set Acute cholecystitis and cholelithiasis CD 1.14 Care Transition- Problem Value Set Acute cholecystitis and cholelithiasis CD 1.14 Care Transition- Problem Value Set laparascopic cholecystectomy The Hasson cannula was reinserted and the remaining port sites inspected and removed under direct vision CE ED Patient Contact Information Definition Main contact information for the patient, including telecommunications and physical addresses. Also includes information on if the patient has a Directspecific electronic endpoint address and has text messaging enabled. Patient Contact Object Summary Clinical Application CDA ID Reference(s) The clinical information that the patient provides about how to reach them. 2.16.840.1.113883.10.20.21.1.1 [US Realm Document Header] Parent Objects Child Objects Demographics Page 62 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Data Element Name Patient DirectEnabled Address Patient Home Address Patient Home Phone Patient Home Phone Text Message Enabled Patient Portal/PHR Available Patient Portal/PHR URL Patient Work Phone Patient Work Phone Text Message Enabled Primary Email Address Secondary Email Patient Contact Information Object in Detail ISO/HL7 Data Element Definition Clinical Example Data Type The electronic endpoint address of the TN patient CDA Reference The current address of the individual to which the exchange refers. Multiple addresses are allowed and the work address may be a method of disclosing the employer AD Person Address A telephone number (voice or fax), TN Patient Phone/Email/URL Is text messaging enabled on the patient's home phone? BL Is a patient portal or PHR available? BL The URL of the patient portal or URI of the PHR TN A telephone number (voice or fax), TN Is text messaging enabled on the patient's work phone? BL Primary email address for the patient TN Secondary email address for the TN Expected Value Set 1.8 Care TransitionCountry Value Set 1.13 Care Transition- Postal Code Value Set 1.20 Care Transition- State Value Set Patient Phone/Email/URL Patient Phone/Email/URL Patient Phone/Email/URL Patient Page 63 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Data Element Name Address Patient Contact Information Object in Detail ISO/HL7 Data Element Definition Clinical Example Data Type patient (may be a work-related email address) CDA Reference Expected Value Set Phone/Email/URL Patient Information Definition Information used to specifically help in the identification of the patient. Patient Information Object Summary Clinical Application CDA ID Reference(s) Content identifies the patient 2.16.840.1.113883.10.20.21.1.1 [US Realm Document Header] Parent Objects Child Objects Demographics Patient Information Object in Detail Data Element Name Mothers Maiden Name Patient Date of Birth Patient Administrative Data Element Definition The family name under which the Mother was born The date and time of birth of the individual to which this Exchange refers. The date of birth is typically a key patient identifier variable and used to enable computation of age at the effective date of any other data element. It is assumed to be unique and fixed throughout the patient's lifetime Gender (i.e., the behavioral, cultural, or psychological traits typically associated Clinical Example ISO/HL7 Data Type CDA Reference PN Mother’s Maiden Name TS Person Date of Birth CE Gender Expected Value Set Page 64 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Patient Information Object in Detail Data Element Name Gender Patient Identifiers Patient Marital Status Patient Name Data Element Definition with one sex) as defined for administrative purposes An identifier that uniquely identifies the individual to which the exchange refers and connects that document to the individual's personal health record. Potential security risks associated with use of SSN or driver's license for this element suggest that these should not be used routinely A value representing the domestic partnership status of a person. Marital status is important in determining insurance eligibility and other legal arrangements surrounding care. Marital status often changes during a patient's lifetime so the data should relate to the effective date of the patient data object and not be entered with multiple values like an address or contact number. This element should only have one instance reflecting the current status of the individual at the time the Exchange is produced. Former values might be part of the personal and social history The individual to whom the exchange refers. Multiple names are allowed to Clinical Example Married Polygamous; Civil Union; Single; Divorced; Widowed ISO/HL7 Data Type CDA Reference II Person ID CE Marital Status PN Person Name Expected Value Set Page 65 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Patient Information Object in Detail Data Element Name Data Element Definition Clinical Example ISO/HL7 Data Type CDA Reference Expected Value Set retain birth name, maiden name, legal names and aliases as required Patient Instructions Definition Information provided to the patient by the care team members detailing what the patient needs to do regarding their healthcare. Patient Instructions Object Summary Clinical Application CDA ID Reference(s) The patient’s wound care instructions including washing the wound daily with warm soapy water, drying the area completely, applying a film of petroleum jelly over the wound and applying a fresh bandage loosely to cover the wound. Parent Objects Child Objects Patient Instructions Object in Detail Data Element Name Patient Instructions Narrative Instruction Delivery Method Instructions Type Definition Records instructions given to a patient. Manner in which instructions were provided Defines the application of instructions. Clinical Example ISO/HL7 Data Type Drink at least 8, 8 oz, glasses of H2O per day ED Verbal ED Patient Education CD CDA Reference Expected Value Set Page 66 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Payer Information Payer Information Object Summary Clinical Application CDA ID Reference(s) Definition Primary and secondary insurance provider information applicable to the patient. 2.16.840.1.113883.10.20.22.2.18 Parent Objects Consultation Request including Clinical Summary Consultation Summary Discharge Summary Child Objects CDA Reference Expected Value Set Payer Information Object in Detail Data Element Name Definition Clinical Example ISO/HL7 Data Type Name of Payer The name of the insurance company EN Insurance Company Name Patient Member ID The identifier assigned by the health insurance payer to the patient INT Member ID Patient Relationship to Subscriber Specifies only if patient is the subscriber or dependent is within the context of the specified health plan CE Patient Relationship to Subscriber Primary Payer Address The official mailing address to which written correspondence is to be directed AD The policy or group contract number ED Primary Payer 1.22 Health Insurance Subscriber Relationship Value Set Health Plan Insurance Information Source Address Group Number Page 67 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Payer Information Object in Detail Data Element Name Group Number Primary Payer Type Definition Clinical Example ISO/HL7 Data Type CDA Reference Expected Value Set identifying the contract between a health plan sponsor and the health plan. This is not a number that uniquely identifies either the subscriber or person covered by the health insurance The type of primary health plan covering the individual Health Insurance Type PPO, HMO, POS, etc. Secondary Payer Address The official mailing address to which written correspondence is to be directed AD Health Plan Insurance Information Source Address Secondary Payer Group Number The policy or group contract number identifying the contract between a health plan sponsor and the health plan. This is not a number that uniquely identifies either the subscriber or person covered by the health insurance ED Group Number TN Health Plan Insurance Information Source Phone/Email/URL Secondary Payer Phone Secondary payer’s contact number Secondary Payer Type The type of secondary health plan covering the individual PPO, HMO, POS, etc. Health Insurance Type 1.23 Health Insurance Type Value Set 1.23 Health Insurance Type Value Set Page 68 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Physical Activity Definition The provider recommended physical activity to the patient. Physical Activity Object Summary Clinical Application CDA ID Reference(s) Parent Objects Child Objects Patient to ambulate using a 4 point walker Physical Activity Object in Detail Data Element Name Data Element Definition Activity Motivation Patient’s perception and/or willingness that they need to increase their exercise intervals, intensity or total weekly commitment to physical activity. Exercise Vital Sign (EVS) Physical Activity Assessment A computable value which translates into “minutes/week” of physical activity. A physical activity assessment is an evaluation of a person's body movement that works muscles and uses more energy than when at rest or that enhances or maintains physical Clinical Example Example: “Yes, I need to increase my activity/exercise” Answer: Yes/No/Unsure Example: 1. Patient states they typically exercise 4 days/week. 2. Patient states they typically walk 15 minutes/day. EHR Calculates: days x minutes/day = minutes/week. Assessment can be completed in terms of frequency, duration, intensity, and type of activity using objective or ISO/HL7 Data Type CDA Reference Expected Value Set BL IVL_TS LIST Page 69 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Physical Activity Object in Detail Data Element Name Data Element Definition fitness and overall health Clinical Example ISO/HL7 Data Type CDA Reference Expected Value Set self-reported measures. Physical Exam Definition Physical examination or clinical examination is the process by which a doctor investigates the body of a patient for signs of disease. It generally follows the taking of the medical history — an account of the symptoms as experienced by the patient. Together with the medical history, the physical examination aids in determining the correct diagnosis and devising the treatment plan. Physical Exam Object Summary Clinical Application CDA ID Reference(s) Parent Objects Pupils equal reactive to light and accommodation; equal ocular movements Intact, 2+ lower extremity edema; Heart: regular rate and rhythm. 2.16.840.1.113883.10.20.22.2.19 Child Objects Consultation Request including Clinical Summary Consultation Summary Discharge Summary Physical Exam Object in Detail Data Element Name Physical Exam Component Data Element Definition Device used by the clinician to make observation Clinical Example Stethoscope ISO/HL7 Data Type CDA Reference Expected Value Set Structure Page 70 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Physical Exam Object in Detail Data Element Name Data Element Definition Clinical Example Physical Exam Narrative Direct observations made by the clinician Pupils equal round and reactive to light and accomodation; The examination may be reported as a collection of random clinical statements or it may be reported categorically Physical Observations Observations made by the examining clinician using inspection, palpation, auscultation, and percussion All normal to examination ISO/HL7 Data Type HIST CE CDA Reference Expected Value Set Primary Care and Designated Providers Definition A list of the primary care physicians applicable to the patient, as well as other designated providers and specialists who may work with the patient. Data Element Name Provider Domain Primary Care and Desginated Providers Object Summary Clinical Application CDA ID Reference(s) This list will include information about the provider's specializations and whether they are part of the patient's care team. 2.16.840.1.113883.10.20.21.1.1 [US Realm Document Header] Primary Care and Designated Providers Object ISO/HL7 Data Element Definition Clinical Example Data Type Provider role uses a coded value to Behavioral Health & Social CF Parent Objects Child Objects Demographics CDA Reference Provider Role Expected Value Set 1.15 Care Page 71 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Data Element Name of Management Provider Fax Number Provider Name Provider NPI Provider Patient Identifier Provider PCMH Provider Phone Number Provider Portal URL Provider Primary Primary Care and Designated Providers Object ISO/HL7 Data Element Definition Clinical Example Data Type classify providers according to the role Service Providers they play in the healthcare of the patient and comes from a very limited set of values. The purpose of this data element is to express the information often required during patient registration, identifying the patient's primary care provider, the referring physician or other consultant involved in the care of the patient The fax number of the provider’s TN organization The name of the provider National Provider Identifier or NPI is a unique identification number issued to healthcare providers in the United States The identifier used by the provider to identify the patient’s medical record PN II Expected Value Set Transition- Provider Role Value Set Provider Phone/Email/URL Encounter Provider National Provider ID II The patient's PCMH EN The provider’s contact phone number TN The URL of the provider’s patient portal The mailing address to which written CDA Reference TN AD Provider’s Organization Name Provider Phone/Email/URL Provider Phone/Email/URL Provider Address 1.8 Care TransitionPage 72 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Data Element Name Address Provider Primary Email Address Primary Care and Designated Providers Object ISO/HL7 Data Element Definition Clinical Example Data Type correspondence to this provider should be directed The primary email for contact purposes Provider Secondary Address The mailing address for the provider if the primary address is unavailable. Provider Specialties Provider type classifies providers according to the type of license or accreditation they hold or the service they provide physician, dentist, pharmacist, etc. CDA Reference Expected Value Set Country Value Set 1.13 Care Transition- Postal Code Value Set 1.20 Care Transition- State Value Set TN Provider Phone/Email/URL TN 1.8 Care TransitionCountry Value Set 1.13 Care Provider Transition- Postal Phone/Email/URL Code Value Set 1.20 Care Transition- State Value Set CF 1.16 Care Transition- Provider Type Value Set Provider Type Problems List Definition What clinician sending the message has determined to be the patient's active Problems List Object Summary Clinical Application CDA ID Reference(s) All of the chronic problems or health issues that the patient’s 2.16.840.1.113883.10.20.22.2.7 treating clinicians have determined Parent Objects Consultation Request including Child Objects Page 73 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Definition problems and/or diagnoses or determination of no known problems - this list may be reconciled at each care transition. The metadata for the problem list is to include: the clinician that assigned the problem to the problem list with the date/time stamp of when the problem was assigned, the start date or onset of the problem, whether or not the problem list was reconciled during this encounter and if so by whom, and whether any problems were changed during this encounter. Problems List Object Summary Clinical Application CDA ID Reference(s) to be chronic noteworthy problems (e.g. this list may include chronic health problems like chronic obstructive pulmonary disease as well as problems such as tobacco use disorder). Parent Objects Clinical Summary Consultation Summary Discharge Instructions Discharge Summary Encounters Child Objects Problems List Object in Detail Data Element Name Active Problem Attributes Active Problem Name Data Element Definition Clinical Example ISO/HL7 Data Type CDA Reference Expected Value Set 1.14 Care TransitionProblem Value Set List of coded values capturing problem health status Asthma CD Problem Code Actual name of the problem No known problems ED Problem Name Page 74 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Problems List Object in Detail Data Element Name Active Problem Type Problem Assignee Problem Assignee ID Reconciled By Reconciliation Date Reconciliation Status Start Date Of Problem Data Element Definition Indicates the level of medical judgment used to determine the existence of a problem The person that entered the problem in the EHR date/time stamped Identifier for the person that entered the problem in the EHR Who reconciled the problem list The date/time stamp for the last reconciliation of the problem list Clinical Example Complaint ISO/HL7 Data Type CDA Reference Expected Value Set CD Problem Type 1.33 Problem Type Value Set PN II Treating Provider Treating Provider ID PN TS Has the problem list been reconciled? Boolean This is the range of time of which the problem was active for the patient Includes the date of onset IVL_TS Problem Date Reason for Consult Request Definition The reason that one physician or other clinical professional is asking for the specialty opinion or action of another physician or other clinical professional. This generally includes context specific patient Reason for Consult Request Object Summary Clinical Application CDA ID Reference(s) Parent Objects The patient has a large left sided thyroid nodule; please evaluate and perform a fine needle aspiration if deemed appropriate. 1.3.6.1.4.1.19376.1.5.3.1.3.1 Child Objects Consultation Request including Clinical Summary Consultation Summary Consult(s) Page 75 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Definition history and the issues that the requesting physician wants the consulting physician to address, or the activities that the requesting physician or other clinical professional wants the consulting physician or other clinical professional to perform. Data Element Name Provisional Diagnosis Requested Procedure Reason for Consult Request Object Summary Clinical Application CDA ID Reference(s) Reason for Consult Request Object in Detail ISO/HL7 Data Element Definition Clinical Example Data Type Description of unconfirmed diagnosis A procedure that is requested as part of this order. Request Reason Reason for consult/procedure encounter request Request Type Indicates the type of request Sore throat or back pain FN Interactive psychiatric diagnostic interview examination A of the thyroid nodule Examples are "Medical Necessity", "Patient's Request" and "Dependency". Note: medical reason(s) for the consult are specified as associated diagnoses. Consult, procedure Parent Objects Assessment(s) and Plan(s) Recommendations Child Objects CDA Reference Expected Value Set CE Procedure Type 1.34 Procedure Value Set CE Reason for Visit ED ED Page 76 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Data Element Name Source of Request Reason for Consult Request Object in Detail ISO/HL7 Data Element Definition Clinical Example Data Type Identifies the source of the consult Community, Other facility EN request CDA Reference Expected Value Set Facility Name Review of Systems Definition Subjective patient supplied information regarding the patient's different bodily systems. Review of Systems Object Summary Clinical Application CDA ID Reference(s) Patient denies change in bowel habits, black stool or bright red 1.3.6.1.4.1.19376.1.5.3.1.3.18 blood per rectum. Parent Objects Child Objects Discharge Summary Review of Systems Object in Detail Data Element Name Data Element Definition Review of Systems Narrative Relevant collection of symptoms and functions systematically gathered by a clinician. Includes symptoms the patient is currently experiencing, some of which were not elicited during the history of present illness, as well as a potentially large number of pertinent negatives, for example, symptoms that the patient denied experiencing. Clinical Example ISO/HL7 Data Type CDA Reference Expected Value Set Patient denies recent history of fever or malaise. Positive for weakness and shortness of breath. One episode of melena. No ED recent headaches. Positive for osteoarthritis in hips, knees and hands. Page 77 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Social History Definition Subjective patient supplied information that addresses occupational and recreational aspects of the patient's personal life that have the potential to be clinically significant such as sexual history, smoking history and ETOH. Social History Object Summary Clinical Application CDA ID Reference(s) Patient smokes 2 packs of cigarettes per day for 20 years. 2.16.840.1.113883.10.20.22.2.17 Parent Objects Consultation Request including Clinical Summary Consultation Summary Discharge Summary Child Objects Social History Object in Detail Data Element Name Social History Details Social History Attribute Status Social History Range Social History Type Data Element Definition Clinical Example Narrative description of social situation. Describes current state of social attribute Range of time the social attribute was active for the patient Active Coded entry for type of social attribute Alcohol intake (observable entity) ISO/HL7 Data Type ED ST IVL_TS CD CDA Reference Expected Value Set Social History Free Text Social History Observed Value Social History Date Social History Type 1.41 Social History Type Value Set Support Contacts Definition A list of the primary and Support Contacts Object Summary Clinical Application CDA ID Reference(s) 2.16.840.1.113883.10.20.21.1.1 Parent Objects Demographics Child Objects Page 78 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Definition secondary caregiver contacts and their relevant information. Support Contacts Object Summary Clinical Application CDA ID Reference(s) [US Realm Document Header] Parent Objects Child Objects Support Contacts Object in Detail Data Element Name Data Element Definition Contact Type Identifies the relationship of the support contact to the patient Primary Emergency Contact Address Primary Emergency Contact Name Primary Emergency Contact Phone Primary Emergency Contact Relationship Secondary Emergency Contact Address Clinical Example CDA Reference CE Contact Type AD Contact Address PN Contact Name Phone number of the primary emergency contact TN Contact Phone/Email/URL Identifies the relationship of the contact person to the individual for which this exchange refers ED Contact Relationship The address of the contact individual or organization providing support to the individual for which this exchange is AD Contact Address The address of the contact individual or organization providing support to the individual for which this exchange is produced The name of the individual or organization providing support to the individual for which this exchange is produced Next of kin ISO/HL7 Data Type Expected Value Set 1.18 Care TransitionRelationship Value Set Page 79 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Support Contacts Object in Detail Data Element Name Secondary Emergency Contact Name Secondary Emergency Contact Phone Secondary Emergency Contact Relationship Data Element Definition produced The name of the individual or organization providing support to the individual for which this exchange is produced Clinical Example ISO/HL7 Data Type CDA Reference PN Contact Name Phone number of secondary emergency contact TN Contact Phone/Email/URL Identifies the relationship of the contact person to the individual for which this exchange refers ED Contact Relationship Expected Value Set Surgical/Procedural History Definition The previous surgery and procedures that a patient has had. Surgical/Procedural History Object Summary Clinical Application CDA ID Reference(s) Patient had cholecystectomy in 1995. 2.16.840.1.113883.10.20.22.2.7 Parent Objects Consultation Request including Clinical Summary Consultation Summary Discharge Summary Child Objects Page 80 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Data Element Name Procedure History Surgical History Surgical/Procedural History Object in Detail ISO/HL7 Data Element Definition Clinical Example Data Type Listing of the patient’s procedure HIST history Listing of the patient’s surgical history HIST CDA Reference Expected Value Set Women’s Health Definition Pertinent information of women's health case summarization of a patient Women’s Health Object Summary Clinical Application CDA ID Reference(s) Captures information of health history specific to women including, scheduled or anticipated tests and studies such as mammograms, pap tests, etc. Parent Objects Child Objects Women’s Health Object in Detail Data Element Name Pregnancy Status History of Sexual Trauma Pap Regimen Pap Regimen Start Date Genetic Relative Data Element Definition Indicates whether the patient is currently pregnant. Indicates whether the patient has experienced any sexual trauma (rape, sexual assault, etc.) as a civilian. Identifies the current Pap regimen for the patient. The date on which the patient began or will begin her current PAP regimen. Indicates whether the patient or the Clinical Example Possible values include: Yes, No, Declined to answer, Unknown. ISO/HL7 Data Type CDA Reference ED Pregnancy Expected Value Set CS CF TS Possible values include: Structure Page 81 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Women’s Health Object in Detail Data Element Name Breast Cancer Status Data Element Definition one of the patient's relatives have had breast cancer. Identifies the current or next breast Breast Treatment procedure or treatment recommended for this patient. The date when the current or next Breast Treatment breast procedure or treatment should Date be completed. Identifies the current or next cervical Cervical procedure or treatment recommended Treatment for this patient. The date when the current or next Cervical cervical procedure or treatment should Treatment Date be completed. An indication of whether the patient DES Daughter was exposed to diethylstilbestrol (DES) in utero. Clinical Example ISO/HL7 Data Type CDA Reference Expected Value Set No family history, 2nd degree relative, 1st degree relative, 3: More than one 1st degree relatives, Personal history, or Unknown. Structure TS Structure TS Possible values include: Yes, No, or Unknown. Structure Vital Signs Definition Vital signs are measures of various physiological Vital Signs Object Summary Clinical Application CDA ID Reference(s) Patient's blood pressure is 120/80, 2.16.840.1.113883.10.20.22.2.4.1 Temp 99 F, Height 5’ 3”, Weight 2.16.840.1.113883.10.20.22.2.4 Parent Objects Consultation Request Child Objects Page 82 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Definition statistics, often taken by health professionals, in order to assess the most basic body functions. Vital Signs Object Summary Clinical Application CDA ID Reference(s) 113, Respiratory Rate 14 and Heart Rate 60. Parent Objects including Clinical Summary Consultation Summary Discharge Summary Child Objects ISO/HL7 Data Type CDA Reference Expected Value Set CE Site Vital Signs Object in Detail Data Element Name Body Site Device Observation Method Data Element Definition Indicates the anatomical site - intended to be specified as left arm, right arm, left leg, etc. May also indicate whether patient is sitting, standing, supine. Identifies the device used to measure the vital sign. Indicates Medical Equipment Object A code that provides additional detail about the means or technique used to ascertain the observation. Observation Range Reference range for the vital sign observation Observation Time The date/time on which the measurement was taken. Provides an indication of the state of the patient at the time of the Patient State Clinical Example 1.6 Care TransitionBody Site Value Set Structure CK IVL TS Standing blood pressure can be significantly Vital Sign Result Reference Range Vital Sign Result Date/Time ED Page 83 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Vital Signs Object in Detail Data Element Name Data Element Definition observation. For example, a blood pressure may be taken while the patient is exercising or at rest. Status Vital Sign ID Vital Sign Type Indicates the status of the Vital Signs measurement record Uniquely identifies the Vital Signs measurement. Indicates which Vital Sign was measured. From a code set of allowable Vital Sign codes. Clinical Example ISO/HL7 Data Type CDA Reference Expected Value Set different from supine and may, for example be an indication of a medication side effect as some blood pressure medications can cause a dangerous drop in blood pressure on standing which could cause falls and injury. CS II Heart Beat CK Vital Sign Result Status Vital Sign Result ID Vital Sign Result Type 1.42 Vital Signs Result Type Page 84 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Appendix A: CEDD Object Model Examples Demographics Object Model Example Patient Contact Information Primary Care Physicians and Designated Providers Patient Home Address: AD (Address) Patient Home Phone: TN (Telephone Number) Secondary Email Address: TN (Telephone Number) Patient Work Phone: TN (Telephone Number) Patient Cell Phone: TN (Telephone Number) Primary Email Address: TN (Telephone Number) Direct Address: TN (Telephone Number) Patient Portal/PHR Available: BL (Boolean) Patient Portal/PHR URL: TN (Telephone Number) Patient Home Phone Text Message Enabled: BL (Boolean) Patient Cell Phone Text Message Enabled: BL (Boolean) Patient Work Phone Text Message Enabled: BL (Boolean) Designated Providers Specialties: CF (Coded element with formatted values) Designated Providers Names: PN (Person Name) Designated Provider NPI: II (Instance Identifier) Designated Provider Contact Information: XAD (Extended Address) Designated Provider Domain of Management: CF (Coded element with formatted values) Designated Provider PCMH: EN (Entity Name) Culturally Sensitive Patient Care Payer Information D e mo g ra p hic s Primary Payer Information: ED (Encapsulated Data) Secondary Payer Information: ED (Encapsulated Data) Race: CE (Coded element) Ethnicity: CE (Coded element) Religion: CE (Coded element) Language: CE (Coded element) Disability: CE (Coded element) Educational Level: CE (Coded element) ID: II (Instance Identifier) Existence of Advanced Directives Advanced Directives: BL (Boolean) Patient Information Gender: CE (Coded element) Patient Name: PN (Person Name) Patient Identifiers: II (Instance Identifier) Mothers Maiden Name: PN (Person Name) Marital Status: CE (Coded element) Date of Birth: DATE Support Contacts Primary Emergency Contact Name: PN (Person Name) Primary Emergency Contact Relationship: CE (Coded element) Primary Emergency Contact Information: AD (Address) Secondary Emergency Contact Name: PN (Person Name) Secondary Emergency Contact Relationship: CE (Coded element) Secondary Emergency Contact Information: AD (Address) Page 85 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Consultation Request including Clinical Summary Object Model Example Culturally Sensitive Patient Care Existence of Advanced Directives Advanced Directives: BL (Boolean) Payer Information Race: CE (Coded element) Ethnicity: CE (Coded element) Religion: CE (Coded element) Language: CE (Coded element) Disability: CE (Coded element) Educational Level: CE (Coded element) Active Medication List Primary Payer Information: ED (Encapsulated Data) Secondary Payer Information: ED (Encapsulated Data) Support Contacts Primary Emergency Contact Name: PN (Person Name) Primary Emergency Contact Relationship: CE (Coded element) Primary Emergency Contact Information: AD (Address) Secondary Emergency Contact Name: PN (Person Name) Secondary Emergency Contact Relationship: CE (Coded element) Secondary Emergency Contact Information: AD (Address) Co ns ulta tio n R e q ue s t inc lud ing Clinic a l Summa ry Patient Contact Information Consultation Request ID: II (Instance Identifier) Active Medication List: LIST (Sequence) Date Of Reconciliation: DATE Status of Reconciliation: CE (Coded element) Reconciled By: EN (Entity Name) Discontinued Medications: CE (Coded element) Changed Medications: CE (Coded element) Medication Code: CE (Coded element) Dose: PQ Frequency: IVL_TS When to Take: IVL_TS Duration: IVL (Interval) Route: CE (Coded element) Patient Instructions: ED (Encapsulated Data) Start Date: DATE Stop Date: DATE Prescriber: EN (Entity Name) Associated Assessment: BAG (Bag) Allergies and Intolerances Patient Home Address: AD (Address) Patient Home Phone: TN (Telephone Number) Secondary Email Address: TN (Telephone Number) Patient Work Phone: TN (Telephone Number) Patient Cell Phone: TN (Telephone Number) Primary Email Address: TN (Telephone Number) Direct Address: TN (Telephone Number) Patient Portal/PHR Available: BL (Boolean) Patient Portal/PHR URL: TN (Telephone Number) Patient Home Phone Text Message Enabled: BL (Boolean) Patient Cell Phone Text Message Enabled: BL (Boolean) Patient Work Phone Text Message Enabled: BL (Boolean) Medication Intolerance: ED (Encapsulated Data) All Environmental Allergens: CE (Coded element) All Food Allergens: CE (Coded element) Reaction Attributes: ED (Encapsulated Data) Reaction Date: TIMESTAMP() Severity of Intolerance or Allergy: CE (Coded element) Reaction Identified By: EN (Entity Name) A/I Attributes: ED (Encapsulated Data) List of Reactions: LIST (Sequence) Patient Information Primary Care Physicians and Designated Providers Active Problem List Discontinued Medications Designated Providers Specialties: CF (Coded element with formatted values) Designated Providers Names: PN (Person Name) Designated Provider NPI: II (Instance Identifier) Designated Provider Contact Information: XAD (Extended Address) Designated Provider Domain of Management: CF (Coded element with formatted values) Designated Provider PCMH: EN (Entity Name) Discontinued Medication List: LIST (Sequence) Gender: CE (Coded element) Patient Name: PN (Person Name) Patient Identifiers: II (Instance Identifier) Mothers Maiden Name: PN (Person Name) Marital Status: CE (Coded element) Date of Birth: DATE Start Date Of Problem: DATE Problem Assignee: EN (Entity Name) Active Codes: CS (Coded Simple Value) Reconciliation Status: CE (Coded element) Reconciliation Date: DATE Reconciled By: EN (Entity Name) Page 86 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Consultation Summary Object Model Example Patient Contact Information Culturally Sensitive Patient Care Existence of Advanced Directives Advanced Directives: BL (Boolean) Race: CE (Coded element) Ethnicity: CE (Coded element) Religion: CE (Coded element) Language: CE (Coded element) Disability: CE (Coded element) Educational Level: CE (Coded element) Patient Home Address: AD (Address) Patient Home Phone: TN (Telephone Number) Secondary Email Address: TN (Telephone Number) Patient Work Phone: TN (Telephone Number) Patient Cell Phone: TN (Telephone Number) Primary Email Address: TN (Telephone Number) Direct Address: TN (Telephone Number) Patient Portal/PHR Available: BL (Boolean) Patient Portal/PHR URL: TN (Telephone Number) Patient Home Phone Text Message Enabled: BL (Boolean) Patient Cell Phone Text Message Enabled: BL (Boolean) Patient Work Phone Text Message Enabled: BL (Boolean) Support Contacts Primary Emergency Contact Name: PN (Person Name) Primary Emergency Contact Relationship: CE (Coded element) Primary Emergency Contact Information: AD (Address) Secondary Emergency Contact Name: PN (Person Name) Secondary Emergency Contact Relationship: CE (Coded element) Secondary Emergency Contact Information: AD (Address) Co nsulta tio n Summa ry Consultation Summary ID: II (Instance Identifier) Active Medication List: LIST (Sequence) Date Of Reconciliation: DATE Status of Reconciliation: CE (Coded element) Reconciled By: EN (Entity Name) Discontinued Medications: CE (Coded element) Changed Medications: CE (Coded element) Medication Code: CE (Coded element) Dose: PQ Frequency: IVL_TS When to Take: IVL_TS Duration: IVL (Interval) Route: CE (Coded element) Patient Instructions: ED (Encapsulated Data) Start Date: DATE Stop Date: DATE Prescriber: EN (Entity Name) Associated Assessment: BAG (Bag) Allergies and Intolerances Medication Intolerance: ED (Encapsulated Data) All Environmental Allergens: CE (Coded element) All Food Allergens: CE (Coded element) Reaction Attributes: ED (Encapsulated Data) Reaction Date: TIMESTAMP() Severity of Intolerance or Allergy: CE (Coded element) Reaction Identified By: EN (Entity Name) A/I Attributes: ED (Encapsulated Data) List of Reactions: LIST (Sequence) Patient Information Gender: CE (Coded element) Patient Name: PN (Person Name) Patient Identifiers: II (Instance Identifier) Mothers Maiden Name: PN (Person Name) Marital Status: CE (Coded element) Date of Birth: DATE Primary Care Physicians and Designated Providers Active Medication List Active Problem List Payer Information Designated Providers Specialties: CF (Coded element with formatted values) Designated Providers Names: PN (Person Name) Designated Provider NPI: II (Instance Identifier) Designated Provider Contact Information: XAD (Extended Address) Designated Provider Domain of Management: CF (Coded element with formatted values) Designated Provider PCMH: EN (Entity Name) Start Date Of Problem: DATE Problem Assignee: EN (Entity Name) Active Codes: CS (Coded Simple Value) Reconciliation Status: CE (Coded element) Reconciliation Date: DATE Reconciled By: EN (Entity Name) Primary Payer Information: ED (Encapsulated Data) Secondary Payer Information: ED (Encapsulated Data) Page 87 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Discharge Instructions Object Model Example Active Medication List Active Medication List: LIST (Sequence) Date Of Reconciliation: DATE Status of Reconciliation: CE (Coded element) Reconciled By: EN (Entity Name) Discontinued Medications: CE (Coded element) Changed Medications: CE (Coded element) Medication Code: CE (Coded element) Dose: PQ Frequency: IVL_TS When to Take: IVL_TS Duration: IVL (Interval) Route: CE (Coded element) Patient Instructions: ED (Encapsulated Data) Start Date: DATE Stop Date: DATE Prescriber: EN (Entity Name) Associated Assessment: BAG (Bag) Existence of Advanced Directives Advanced Directives: BL (Boolean) D is c ha rg e Ins truc tio ns Discharge Instructions ID: II (Instance Identifier) Allergies and Intolerances Active Problem List Start Date Of Problem: DATE Problem Assignee: EN (Entity Name) Active Codes: CS (Coded Simple Value) Reconciliation Status: CE (Coded element) Reconciliation Date: DATE Reconciled By: EN (Entity Name) Medication Intolerance: ED (Encapsulated Data) All Environmental Allergens: CE (Coded element) All Food Allergens: CE (Coded element) Reaction Attributes: ED (Encapsulated Data) Reaction Date: TIMESTAMP() Severity of Intolerance or Allergy: CE (Coded element) Reaction Identified By: EN (Entity Name) A/I Attributes: ED (Encapsulated Data) List of Reactions: LIST (Sequence) Page 88 of 89 Version 2.0 Office of the National Coordinator for Health IT Standards & Interoperability Framework ToC CEDD Discharge Summary Object Model Example Active Medication List Active Medication List: LIST (Sequence) Date Of Reconciliation: DATE Status of Reconciliation: CE (Coded element) Reconciled By: EN (Entity Name) Discontinued Medications: CE (Coded element) Changed Medications: CE (Coded element) Medication Code: CE (Coded element) Dose: PQ Frequency: IVL_TS When to Take: IVL_TS Duration: IVL (Interval) Route: CE (Coded element) Patient Instructions: ED (Encapsulated Data) Start Date: DATE Stop Date: DATE Prescriber: EN (Entity Name) Associated Assessment: BAG (Bag) Allergies and Intolerances Medication Intolerance: ED (Encapsulated Data) All Environmental Allergens: CE (Coded element) All Food Allergens: CE (Coded element) Reaction Attributes: ED (Encapsulated Data) Reaction Date: TIMESTAMP() Severity of Intolerance or Allergy: CE (Coded element) Reaction Identified By: EN (Entity Name) A/I Attributes: ED (Encapsulated Data) List of Reactions: LIST (Sequence) Is Contained In / Contains D is c ha rg e S umma ry Is Contained In / Contains Discharge Summary ID: II (Instance Identifier) Is Contained In / Contains Active Problem List Start Date Of Problem: DATE Problem Assignee: EN (Entity Name) Active Codes: CS (Coded Simple Value) Reconciliation Status: CE (Coded element) Reconciliation Date: DATE Reconciled By: EN (Entity Name) Page 89 of 89 Version 2.0