Energy Band Diagrams - Lane Department of Computer Science

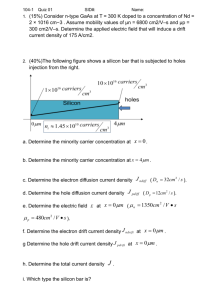
Overview of Silicon Device Physics
Dr. David W. Graham West Virginia University Lane Department of Computer Science and Electrical Engineering 1
Silicon Silicon is the primary semiconductor used in VLSI systems Si has 14 Electrons Energy Bands (Shells) Valence Band Nucleus Silicon has 4 outer shell / valence electrons At T=0K, the highest energy band occupied by an electron is called the valence band.
2
Energy Bands Increasing Electron Energy Energy Bands } Disallowed Energy States } Allowed Energy States • • • Electrons try to occupy the lowest energy band possible Not every energy level is a legal state for an electron to occupy These legal states tend to arrange themselves in bands 3
E C E V Energy Bands E g Energy Bandgap
Conduction Band
First unfilled energy band at T=0K
Valence Band
Last filled energy band at T=0K 4
Band Diagrams E C E V Band Diagram Representation Energy plotted as a function of position E g Increasing electron energy Increasing voltage E C Conduction band Lowest energy state for a free electron E V Valence band Highest energy state for filled outer shells E G Band gap Difference in energy levels between E C and E V No electrons (e ) in the bandgap (only above E C E G = 1.12eV in Silicon or below E V ) 5
Intrinsic Semiconductor Silicon has 4 outer shell / valence electrons Forms into a lattice structure to share electrons 6
Intrinsic Silicon The valence band is full, and no electrons are free to move about E C E V However, at temperatures above T=0K, thermal energy shakes an electron free 7
Semiconductor Properties For T > 0K Electron shaken free and can cause current to flow h + e
–
• • • • • Generation – Creation of an electron (e ) and hole (h + ) pair h + is simply a missing electron, which leaves an excess positive charge (due to an extra proton) Recombination – if an e and an h + when they move, they carry current come in contact, they annihilate each other Electrons and holes are called “carriers” because they are charged particles – Therefore, semiconductors can conduct electricity for T > 0K … but not much current (at room temperature (300K), pure silicon has only 1 free electron per 3 trillion atoms) 8
Doping • Doping – Adding impurities to the silicon crystal lattice to increase the number of carriers • Add a small number of atoms to increase either the number of electrons or holes 9
Column 3 Elements have 3 electrons in the Valence Shell Periodic Table Column 4 Elements have 4 electrons in the Valence Shell Column 5 Elements have 5 electrons in the Valence Shell 10
Donors n-Type Material • • • • • • • • Donors Add atoms with 5 valence-band electrons ex. Phosphorous (P) “Dontates an extra e that can freely travel around Leaves behind a positively charged nucleus (cannot move) Overall, the crystal is still electrically neutral Called “n-type” material (added negative carriers) N D = the concentration of donor atoms [atoms/cm 3 or cm -3 ] ~10 15 -10 20 cm -3 e is free to move about the crystal (Mobility m n ≈1350cm 2 /V) + 11
Donors n-Type Material • • • • • • • • Donors Add atoms with 5 valence-band electrons ex. Phosphorous (P) “Donates” an extra e that can freely travel around Leaves behind a positively charged nucleus (cannot move) Overall, the crystal is still electrically neutral Called “n-type” material (added negative carriers) N D = the concentration of donor atoms [atoms/cm 3 or cm -3 ] ~10 15 -10 20 cm -3 e is free to move about the crystal (Mobility m n ≈1350cm 2 /V) n-Type Material + – + – + – + + – + – + – + – + + – + + – – + – + – – + + – + – + – + – + – + Shorthand Notation Positively charged ion; immobile Negatively charged e-; mobile; Called “majority carrier” Positively charged h+; mobile; Called “minority carrier” 12
Acceptors Make p-Type Material –– h + • • • • • • • • Acceptors Add atoms with only 3 valence band electrons ex. Boron (B) “Accepts” e – and provides extra h + to freely travel around Leaves behind a negatively charged nucleus (cannot move) Overall, the crystal is still electrically neutral Called “p-type” silicon (added positive carriers) N A = the concentration of acceptor atoms [atoms/cm 3 or cm -3 ] Movement of the hole requires breaking of a bond! (This is hard, so mobility is low, μ p ≈ 500cm 2 /V) 13
Acceptors Make p-Type Material p-Type Material – – + + – + – – + – – + + – + – – – + + – – + + – + + – – – + + – + – + – + – Shorthand Notation Negatively charged ion; immobile Positively charged h+; mobile; Called “majority carrier” Negatively charged e-; mobile; Called “minority carrier” • • • • • • • • Acceptors Add atoms with only 3 valence band electrons ex. Boron (B) “Accepts” e – and provides extra h + to freely travel around Leaves behind a negatively charged nucleus (cannot move) Overall, the crystal is still electrically neutral Called “p-type” silicon (added positive carriers) N A = the concentration of acceptor atoms [atoms/cm 3 or cm -3 ] Movement of the hole requires breaking of a bond! (This is hard, so mobility is low, μ p ≈ 500cm 2 /V) 14
The Fermi Function The Fermi Function • Probability distribution function (PDF) • The probability that an available state at an energy E will be occupied by an e -
f
1
e
E
1
E f
kT
E Energy level of interest E k f Fermi level Halfway point Where f(E) = 0.5
Boltzmann constant = 1.38
×10 -23 J/K = 8.617
×10 -5 eV/K T Absolute temperature (in Kelvins) f(E) 1 0.5
E f E 15
Boltzmann Distribution If
E
E f
kT
Then
f
e
E
E f
kT
Boltzmann Distribution • Describes exponential decrease in the density of particles in thermal equilibrium with a potential gradient • Applies to all physical systems • Atmosphere Exponential distribution of gas molecules • • Electronics Exponential distribution of electrons Biology Exponential distribution of ions f(E) 1 0.5
~Ef - 4kT E f ~Ef + 4kT E 16
Band Diagrams (Revisited) E C E g E f E V Band Diagram Representation Energy plotted as a function of position E C E V E f E G Conduction band Lowest energy state for a free electron Electrons in the conduction band means current can flow Valence band Highest energy state for filled outer shells Holes in the valence band means current can flow Fermi Level Shows the likely distribution of electrons Band gap Difference in energy levels between E C and E V No electrons (e ) in the bandgap (only above E C E G = 1.12eV in Silicon or below E V ) E 0.5
1 f(E) • • Virtually all of the valence-band energy levels are filled with e Virtually no e in the conduction band 17
Effect of Doping on Fermi Level E f is a function of the impurity-doping level n-Type Material E E C E f E V 0.5
1 f(E) • • High probability of a free e in the conduction band Moving E f closer to E C majority carriers (higher doping) increases the number of available 18
Effect of Doping on Fermi Level E f is a function of the impurity-doping level p-Type Material 1
f
E C E f E V • • • Low probability of a free e High probability of h + in the conduction band in the valence band Moving E f closer to E V majority carriers (higher doping) increases the number of available 19
Thermal Motion of Charged Particles • • • Applies to both electronic systems and biological systems Look at drift and diffusion in silicon Assume 1-D motion 20
Drift Drift → Movement of charged particles in response to an external field (typically an electric field) E-field applies force
F = qE
which accelerates the charged particle. However, the particle does not accelerate indefinitely because of collisions with the lattice (velocity saturation) Average velocity
electrons holes
µ n µ p
→ electron mobility → empirical proportionality constant between E and velocity → hole mobility
µ n ≈ 3µ p
E 21
Drift Drift → Movement of charged particles in response to an external field (typically an electric field) E-field applies force
F = qE
which accelerates the charged particle. However, the particle does not accelerate indefinitely because of collisions with the lattice (velocity saturation) Average velocity
electrons holes
µ n µ p
→ electron mobility → empirical proportionality constant between E and velocity → hole mobility
µ n ≈ 3µ p
Current Density
J n
,
drift J p
,
drift
m
n qnE
m
p qpE q = 1.6×10 -19
C, carrier density n = number of e p = number of h + 22
Diffusion • • • Diffusion → Motion of charged particles due to a concentration gradient Charged particles move in random directions Charged particles tend to move from areas of high concentration to areas of low concentration (entropy – Second Law of Thermodynamics) Net effect is a current flow (carriers moving from areas of high concentration to areas of low concentration)
J n
,
diff
qD n dn
dx J p
,
diff
qD p dp dx q = 1.6×10 -19
C, carrier density D = Diffusion coefficient n(x) = e density at position x p(x) = h + density at position x → The negative sign in J
p,diff
is due to moving in the opposite direction from the concentration gradient → The positive sign from J
n,diff
is because the negative from the e cancels out the negative from the concentration gradient 23
Einstein Relation Einstein Relation → Relates D and µ (they are not independent of each other)
D
m
kT q U T = kT/q
→ Thermal voltage = 25.86mV at room temperature ≈ 25mV for quick hand approximations → Used in biological and silicon applications 24
p-n Junctions (Diodes) • • • • • p-n Junctions (Diodes) Fundamental semiconductor device In every type of transistor Useful circuit elements (one-way valve) Light emitting diodes (LEDs) Light sensors (imagers) 25
p-n Junctions (Diodes) + – + – + – + – + – + – + – + – + – + – + – + – + – + – + – – + – + – + p-type + – + – + – – + – + – + n-type + – + – + – + – + – + – – + – + – + + – + – + – + – + – + – + – + – + – + – + – + – + – + – + – Bring p-type and n-type material into contact 26
p-n Junctions (Diodes) p-type + + + + + + + + + – + – + – + – + – + – n-type – + – + – + – + – + – + – + – + – + – + – + – + – + – + – + – Depletion Region • • • • All the h + from the p-type side and e from the n-type side undergo diffusion → Move towards the opposite side (less concentration) When the carriers get to the other side, they become minority carriers Recombination → The minority carriers are quickly annihilated by the large number of majority carriers All the carriers on both sides of the junction are depleted from the material leaving • Only charged, stationary particles (within a given region) • A net electric field This area is known as the depletion region (depleted of carriers) 27
Charge Density p-type + + + + + + + + + – + – + – + – + – + – n-type – + – + – + – + – + – + – + – + – + – + – + – + – + – + – + – Depletion Region (x) qN D x -qN A The remaining stationary charged particles results in areas with a net charge 28
(x) qN D -qN A E Electric Field x x • Areas with opposing charge densities creates an E-field • E-field is the integral of the charge density • Poisson’s Equation
dE
dx
ε is the permittivity of Silicon 29
(x) qN D -qN A E bi Potential x x x • E-field sets up a potential difference • Potential is the negative of the integral of the E-field
d
dx
E
30
(x) qN D -qN A E E C E f E V bi Band Diagram x x x • Line up the Fermi levels • Draw a smooth curve to connect them 31
E C E f E V p-type p-n Junction Band Diagram VA p n n-type 32
E C E f E V p-n Junction – No Applied Bias VA If V
A = 0
p n • Any e or h + that wanders into the depletion region will be swept to the other side via the E-field • Some e and h + have sufficient energy to diffuse across the depletion region • If no applied voltage
I drift = I diff
33
E C E f E V p-n Junction – Reverse Biased VA If V
A < 0
p n Reverse Biased • Barrier is increased • No diffusion current occurs (not sufficient energy to cross the barrier) • Drift may still occur • Any generation that occurs inside the depletion region adds to the drift current • All current is drift current 34
E C E f E V p-n Junction – Forward Biased VA If V
A < 0
p n Forward Biased • Barrier is reduced, so more e and h + may diffuse across • Increasing V
A
and h + increases the e that have sufficient energy to cross the boundary in an exponential relationship (Boltzmann Distributions) →Exponential increase in diffusion current • Drift current remains the same 35
p-n Junction Diode
I
I
0
e V A nU T
1 Diffusion Drift Combination of drift and generation
U T
kT q
→ Thermal voltage = 25.86mV
n
1 2 36
p-n Junction Diode I
I
I
0
e V A nU T
1
I
0
e V A
I
0
nU T
ln(I) for V
A > 0
for V
A < 0
1
nU T
q nkT
-I 0
I
I
0
e V A nU T
1
I I
0
e V A nU T
1 V A ln(I 0 ) V A ln
I I
0 ln ln ln
e V A
e V A nU T nU T
ln 0 ln
V A nU T
ln 0 37
Curve Fitting Exponential Data (In MATLAB) Curve Fitting Exponential Data (In MATLAB)
I
I
0
e V A nU T
• Given I and V (vectors of data) • Use the MATLAB functions •polyfit – function to fit a polynomial (find the coefficients) •polyval – function to plot a polynomial with given coefficients and x values [A] = polyfit(V,log(I),1); % polyfit(independent_var,dependent_var,polynomial_order) % A(1) = slope % A(2) = intercept [I_fit] = polyval(A,V); % draws the curve-fit line 38
![Semiconductor Theory and LEDs []](http://s2.studylib.net/store/data/005344282_1-002e940341a06a118163153cc1e4e06f-300x300.png)