What are your responsibilities?
advertisement

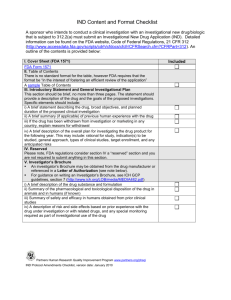
Investigational New Drug Application (IND) What is involved? What are your responsibilities? May 2013: Research Institute, Clinical Research Administration Topics • Definition of IND • Roles and Responsibilities of: • Sponsor • Investigator • Sponsor-Investigator • When an IND needed • When an IND not needed • What is involved with an IND submission • Management of Active IND KUMC Research Institute, 2013 Definition of IND • Provisions of the IND Regulation – 21 CFR 312 • “An investigational new drug for which an IND is in effect in accordance with this part is exempt from premarketing approval requirements…and may be shipped lawfully for purpose of conducting investigation of that drug” • IND is regulatory mechanism for new drug development KUMC Research Institute, 2013 What are roles and responsibilities • Sponsor: a person who takes responsibility for and initiates a clinical investigation. The sponsor may be a/an: • • • • • Individual Pharmaceutical Company Governmental Agency Academic Institution Private or Other Organization • A sponsor does not actually conduct the investigation KUMC Research Institute, 2013 What are roles and responsibilities • Select Sponsor Requirements (21 CFR 312.50): • Select qualified investigators by education and experience • Provide investigators with all information needed to conduct the investigation properly • Monitor the study • Ensure study is conducted according to protocol • Inform FDA and investigators of significant new adverse effects or risks with respect to the drug KUMC Research Institute, 2013 What are roles and responsibilities • Investigator: an individual who actually conducts a clinical investigation (i.e. under whose immediate direction the drug is administered and dispensed to a subject). KUMC Research Institute, 2013 What are roles and responsibilities • Select Investigator Requirements (21 CFR 312.60): • Ensure the investigation is conducted according to: • The signed investigator statement (1572) • The investigational plan • Applicable regulations • Protecting rights, safety and welfare of subject’s under investigator’s care • Control of drugs • Obtain informed consent (unless there is a waiver of consent, see 21 CFR 50.23 or 50.24) KUMC Research Institute, 2013 What are roles and responsibilities • Sponsor-Investigator: an individual who both initiates and conducts an investigation, and under whose immediate direction the drug is administered or dispensed. • Always an individual • Requirements of Sponsor-Investigator include both those applicable to an investigator and a sponsor • This role applicable to many KUMC IND’s. For more information on additional requirements of role, contact the HSC and Research Institute. KUMC Research Institute, 2013 When an IND is needed • A sponsor shall submit an IND to FDA if the sponsor intends to conduct a clinical investigation with an investigational new drug that is subject to 312.2(a) • Submit an IND if any exempt criteria are not met • If you are uncertain if an IND is required, please contact the Research Institute. We will help you with the determination or submit a request to the FDA for a determination. KUMC Research Institute, 2013 When an IND not needed For a study to be exempt from an IND, it must meet ALL six criteria: 1. The investigational drug is lawfully marketed in the United States 2. The investigation is not intended to be reported to the FDA as a well-controlled study in support of a new indication for use of the drug product 3. The investigation is not intended to support a significant change in advertising to an existing lawfully marketed prescription drug product 4. The investigation does not involve a route or administration or dosage level or use in a patient population or other factor that significantly increases the risks (or decreases the acceptability of the risks) associated with the use of the drug product 5. The investigation will be conducted in compliance with the requirements for institutional review set forth in FDA regulations 21 CFR 56, and requirements for informed consent as set forth in FDA regulations 21 CFR 50 6. The investigation will be conducted in compliance with FDA regulations 21 CFR 312.7: Promotion and charging for investigational drugs KUMC Research Institute, 2013 What is involved in IND submission • Content requirements for an IND submission are found in 21 CFR 312.23 • Essential forms for a submission: • 1571: must accompany every submission to the FDA for the IND • 1572: Statement of Investigator • 3674: related to clinicaltrials.gov posting KUMC Research Institute, 2013 What is a 1571? • Required Form for EVERY IND Submission • Details what is included in submission • Identifies Study Information used to Classify the study • Found at: http://www.fda.gov/downloads/aboutfda/reportsmanualsforms /forms/ucm083533.pdf KUMC Research Institute, 2013 What is a 1571? • Important: by signing you agree not to begin any clinical investigations: • “…until 30 days after FDA’s receipt of the IND unless I receive earlier notification by FDA that the studies may begin…” And; • “…covered by the IND if those studies are placed on clinical hold or financial hold” • If you do not hear any response from the FDA for 30 days after the date they receive the submission, the IND is considered “in effect”. KUMC Research Institute, 2013 What is a 1572? • Called the “Statement of Investigator” • Lists Commitments of the Investigator • Provides Required Information to the FDA Criteria 1572 Fulfills for IND: • Location of PI, Study, Labs • Name of Responsible IRB • Names of Sub-Investigators Found at: http://www.fda.gov/downloads/AboutFDA/ReportsManualsForms/Forms/UCM074728.pdf KUMC Research Institute, 2013 What is involved in IND submission • Essential documents for a submission: • Cover letter/Introductory Statement /General Investigational Plan (2-3 pages) • Investigator’s Brochure (if available from manufacturer; not required for single center investigator initiated trial submissions) • Protocol • Draft Informed Consent Form • Cross Reference Letter (provided by manufacturer which gives submission right to reference all previous data related to drug submitted to the FDA – chemistry, pharmacology and toxicology, previous human experience) KUMC Research Institute, 2013 What is involved in IND submission • Depending on the study, there may be additional requirements for the IND submission. • The CRA has experience compiling, submitting and managing IND’s for KUMC Investigators. Your Clinical Trial Project Manager in the CRA will assist you through the entire process. KUMC Research Institute, 2013 What If I Need an IND? • Investigator and CRA a Team for IND Submission • Investigator brings expertise and protocol • CRA brings FDA and IRB submission and approval know how • Together, research moves forward KUMC Research Institute, 2013 Investigator CRA • Protocol • Expertise • Design • FDA Preparation • IRB Preparation What is involved after initial IND submission? • Continuing management of the IND is essential. Submissions to the FDA to keep them appraised of study activity includes: • Annual Reports (Sponsor; 312.33): due within 60 days of IND anniversary date (date the IND went into effect) • Unanticipated Problem Reports (Sponsor): to the FDA and any sub-sites • Revised Protocol (Sponsor): changes in risk/benefit of trial, change that impacts subject safety • Changes in the study team, study sites (Investigator) KUMC Research Institute, 2013