Buffers Made Easy
advertisement

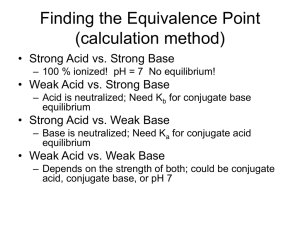
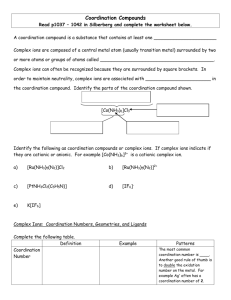
Buffers Made Easy Common ion effect Common Ion: the presence of an ion which appears in both the acid (or base) and a salt in the solution Common ion effect: The shift that occurs because of the addition of an ion already involved in the equilibrium reaction. Buffered Solutions • A solution that resists a change in it’s pH when either hydroxide ions or protons(hydrogen) ions are added. • Consists of a WEAK acid and it’s SALT • Weak acids or bases and a common ion. How does it work • If OH- are added to the system it moves right. Not allowing OH- to accumulate not changing the pH • If H+ are added it will proceed in the reverse to produce HA not allowing the pH to change Look at the Eq expression • The Eq concentration of H+ is determined by the ratio of [HA]/[A-]. Bases are the opposite • You will use Kb Hendersen- Hasselbach Simpler method • One equation: Work buffers as an equilbrium problem Go back to the reaction HA H+ + A• If acid is added to the buffer, simply add acid to the numerator AND subtract the same quantity from the base since it was self-sacrificing and neutralized the acid. If base is added, simply add the base to the denominator and subtract from the numerator. Add or subtract in moles NOT molarity. Moles = molarity x volume • When equal concentrations (or moles) of Acid and Base are present [which occurs at the . equivalence point of a titration] the ratio of acid to base equals ONE and therefore, the pH = pKa. IF you are asked to construct a buffer of a specific pH and given a table of Ka’s, choose a Ka with an exponent close to the desired pH and use equal concentrations of the acid and base RECAP • Go to MOLES. Moles = M x V • Add to what ever is being added and subtract from the other. The titration curve of a strong acid with a strong base. 16.5 Strong Acid-Strong Base Titrations 100% ionization! NaOH (aq) + HCl (aq) OH- (aq) + H+ (aq) H2O (l) + NaCl (aq) No equilibrium H2O (l) 16.4 Weak Acid-Strong Base Titrations CH3COOH (aq) + NaOH (aq) CH3COOH (aq) + OH- (aq) CH3COONa (aq) + H2O (l) CH3COO- (aq) + H2O (l) At equivalence point (pH > 7): CH3COO- (aq) + H2O (l) OH- (aq) + CH3COOH (aq) 16.4 Strong Acid-Weak Base Titrations HCl (aq) + NH3 (aq) H+ (aq) + NH3 (aq) NH4Cl (aq) NH4Cl (aq) At equivalence point (pH < 7): NH4+ (aq) + H2O (l) NH3 (aq) + H+ (aq) 16.4 Acid-Base Indicators 16.5 pH 16.5 Complex Ion Formation • These are usually formed from a transition metal surrounded by ligands (polar molecules or negative ions). • As a "rule of thumb" you place twice the number of ligands around an ion as the charge on the ion... example: the dark blue Cu(NH3)42+ (ammonia is used as a test for Cu2+ ions), and Ag(NH3)2+. • Memorize the common ligands. Common Ligands Ligands Names used in the ion H2O NH3 aqua ammine OHClBrCNSCN- hydroxy chloro bromo cyano thiocyanato (bonded through sulphur) isothiocyanato (bonded through nitrogen) Names • Names: ligand first, then cation Examples: – tetraamminecopper(II) ion: Cu(NH3)42+ – diamminesilver(I) ion: Ag(NH3)2+. – tetrahydroxyzinc(II) ion: Zn(OH)4 2- • The charge is the sum of the parts (2+) + 4(-1)= -2. Coordination Number • Total number of bonds from the ligands to the metal atom. • Coordination numbers generally range between 2 and 12, with 4 (tetracoordinate) and 6 (hexacoordinate) being the most common. Some Coordination Complexes molecular formula Lewis base/ligand Lewis acid donor atom coordination number Ag(NH3)2+ NH3 Ag+ N 2 [Zn(CN)4]2- CN- Zn2+ C 4 [Ni(CN)4]2- CN- Ni2+ C 4 [PtCl6] 2- Cl- Pt4+ Cl 6 Ni2+ N 6 [Ni(NH3)6]2+ NH3 When Complexes Form • Aluminum also forms complex ions as do some post transitions metals. Ex: Al(H2O)63+ • Transitional metals, such as Iron, Zinc and Chromium, can form complex ions. • The odd complex ion, FeSCN2+, shows up once in a while • Acid-base reactions may change NH3 into NH4+ (or vice versa) which will alter its ability to act as a ligand. • Visually, a precipitate may go back into solution as a complex ion is formed. For example, Cu2+ + a little NH4OH will form the light blue precipitate, Cu(OH)2. With excess ammonia, the complex, Cu(NH3)42+, forms. • Keywords such as "excess" and "concentrated" of any solution may indicate complex ions. AgNO3 + HCl forms the white precipitate, AgCl. With excess, concentrated HCl, the complex ion, AgCl2-, forms and the solution clears.