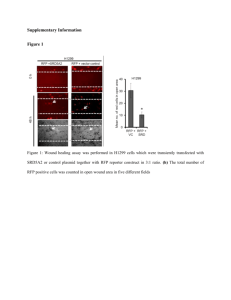
Andrea Marion
advertisement
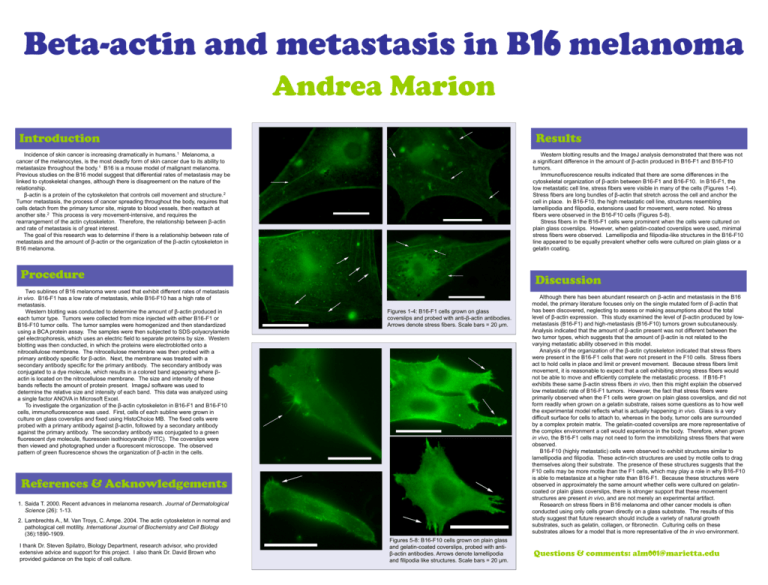
Beta-actin and metastasis in B16 melanoma Andrea Marion Introduction Results Incidence of skin cancer is increasing dramatically in humans.1 Melanoma, a cancer of the melanocytes, is the most deadly form of skin cancer due to its ability to metastasize throughout the body.1 B16 is a mouse model of malignant melanoma. Previous studies on the B16 model suggest that differential rates of metastasis may be linked to cytoskeletal changes, although there is disagreement on the nature of the relationship. β-actin is a protein of the cytoskeleton that controls cell movement and structure. 2 Tumor metastasis, the process of cancer spreading throughout the body, requires that cells detach from the primary tumor site, migrate to blood vessels, then reattach at another site.2 This process is very movement-intensive, and requires the rearrangement of the actin cytoskeleton. Therefore, the relationship between β-actin and rate of metastasis is of great interest. The goal of this research was to determine if there is a relationship between rate of metastasis and the amount of β-actin or the organization of the β-actin cytoskeleton in B16 melanoma. Western blotting results and the ImageJ analysis demonstrated that there was not a significant difference in the amount of β-actin produced in B16-F1 and B16-F10 tumors. Immunofluorescence results indicated that there are some differences in the cytoskeletal organization of β-actin between B16-F1 and B16-F10. In B16-F1, the low metastatic cell line, stress fibers were visible in many of the cells (Figures 1-4). Stress fibers are long bundles of β-actin that stretch across the cell and anchor the cell in place. In B16-F10, the high metastatic cell line, structures resembling lamellipodia and filipodia, extensions used for movement, were noted. No stress fibers were observed in the B16-F10 cells (Figures 5-8). Stress fibers in the B16-F1 cells were prominent when the cells were cultured on plain glass coverslips. However, when gelatin-coated coverslips were used, minimal stress fibers were observed. Lamellipodia and filipodia-like structures in the B16-F10 line appeared to be equally prevalent whether cells were cultured on plain glass or a gelatin coating. Procedure Two sublines of B16 melanoma were used that exhibit different rates of metastasis in vivo. B16-F1 has a low rate of metastasis, while B16-F10 has a high rate of metastasis. Western blotting was conducted to determine the amount of β-actin produced in each tumor type. Tumors were collected from mice injected with either B16-F1 or B16-F10 tumor cells. The tumor samples were homogenized and then standardized using a BCA protein assay. The samples were then subjected to SDS-polyacrylamide gel electrophoresis, which uses an electric field to separate proteins by size. Western blotting was then conducted, in which the proteins were electroblotted onto a nitrocellulose membrane. The nitrocellulose membrane was then probed with a primary antibody specific for β-actin. Next, the membrane was treated with a secondary antibody specific for the primary antibody. The secondary antibody was conjugated to a dye molecule, which results in a colored band appearing where βactin is located on the nitrocellulose membrane. The size and intensity of these bands reflects the amount of protein present. ImageJ software was used to determine the relative size and intensity of each band. This data was analyzed using a single factor ANOVA in Microsoft Excel. To investigate the organization of the β-actin cytoskeleton in B16-F1 and B16-F10 cells, immunofluorescence was used. First, cells of each subline were grown in culture on glass coverslips and fixed using HistoChoice MB. The fixed cells were probed with a primary antibody against β-actin, followed by a secondary antibody against the primary antibody. The secondary antibody was conjugated to a green fluorescent dye molecule, fluorescein isothiocyanate (FITC). The coverslips were then viewed and photographed under a fluorescent microscope. The observed pattern of green fluorescence shows the organization of β-actin in the cells. Discussion Figures 1-4: B16-F1 cells grown on glass coverslips and probed with anti-β-actin antibodies. Arrows denote stress fibers. Scale bars = 20 µm. References & Acknowledgements 1. Saida T. 2000. Recent advances in melanoma research. Journal of Dermatological Science (26): 1-13. 2. Lambrechts A., M. Van Troys, C. Ampe. 2004. The actin cytoskeleton in normal and pathological cell motility. International Journal of Biochemistry and Cell Biology (36):1890-1909. I thank Dr. Steven Spilatro, Biology Department, research advisor, who provided extensive advice and support for this project. I also thank Dr. David Brown who provided guidance on the topic of cell culture. Figures 5-8: B16-F10 cells grown on plain glass and gelatin-coated coverslips, probed with antiβ-actin antibodies. Arrows denote lamellipodia and filipodia like structures. Scale bars = 20 µm. Although there has been abundant research on β-actin and metastasis in the B16 model, the primary literature focuses only on the single mutated form of β-actin that has been discovered, neglecting to assess or making assumptions about the total level of β-actin expression. This study examined the level of β-actin produced by lowmetastasis (B16-F1) and high-metastasis (B16-F10) tumors grown subcutaneously. Analysis indicated that the amount of β-actin present was not different between the two tumor types, which suggests that the amount of β-actin is not related to the varying metastatic ability observed in this model. Analysis of the organization of the β-actin cytoskeleton indicated that stress fibers were present in the B16-F1 cells that were not present in the F10 cells. Stress fibers act to hold cells in place and limit or prevent movement. Because stress fibers limit movement, it is reasonable to expect that a cell exhibiting strong stress fibers would not be able to move and efficiently complete the metastatic process. If B16-F1 exhibits these same β-actin stress fibers in vivo, then this might explain the observed low metastatic rate of B16-F1 tumors. However, the fact that stress fibers were primarily observed when the F1 cells were grown on plain glass coverslips, and did not form readily when grown on a gelatin substrate, raises some questions as to how well the experimental model reflects what is actually happening in vivo. Glass is a very difficult surface for cells to attach to, whereas in the body, tumor cells are surrounded by a complex protein matrix. The gelatin-coated coverslips are more representative of the complex environment a cell would experience in the body. Therefore, when grown in vivo, the B16-F1 cells may not need to form the immobilizing stress fibers that were observed. B16-F10 (highly metastatic) cells were observed to exhibit structures similar to lamellipodia and filipodia. These actin-rich structures are used by motile cells to drag themselves along their substrate. The presence of these structures suggests that the F10 cells may be more motile than the F1 cells, which may play a role in why B16-F10 is able to metastasize at a higher rate than B16-F1. Because these structures were observed in approximately the same amount whether cells were cultured on gelatincoated or plain glass coverslips, there is stronger support that these movement structures are present in vivo, and are not merely an experimental artifact. Research on stress fibers in B16 melanoma and other cancer models is often conducted using only cells grown directly on a glass substrate. The results of this study suggest that future research should include a variety of natural growth substrates, such as gelatin, collagen, or fibronectin. Culturing cells on these substrates allows for a model that is more representative of the in vivo environment. Questions & comments: alm001@marietta.edu