Acid Rain
advertisement

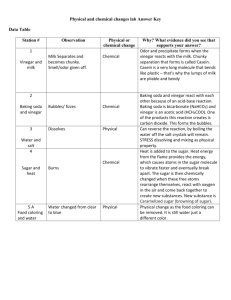
Teko Mmolawa Teacher’s Guide: Understanding the effects of acid rain Created for 5th grade Teaching Objectives: Introduce to students the basic chemistry of acid-base reactions. When acids and bases react, a neutralization reaction occurs, in which both the acid and the base are used up. The reaction gives off carbon dioxide and creates a neutral solution. Through this exercise they will also gain an understanding of the effects of acid rain on the environment. Acid rain affects infrastructure and plant and animal life. Low pH in surface water reduces the amount of plant and animal life. This exercise can also help students understand how factories can be harmful to the environment. Students will investigate which materials get corroded by acids and which ones are resistant. For fun, students could also make volcanoes and add food coloring to them. Key Terms: Acid – a chemical substance that has a pH value less than 7, and neutralizes bases Base – a chemical substance that has a pH value higher than 7 Acid Rain – rain or any other form of precipitation that is unusually acidic pH – a number that shows how acidic or basic a substance is. Materials: Indicator paper 5 liters white vinegar 2 kg baking soda A box chalk Steel wool Detergent Cups (3 per student) Funnels (3 per student) Duct tape Food coloring A wide container (1 per student) Approximate Time: 45 minutes Procedure: 1. 2. 3. 4. Break chalk into smaller pieces. Pour 50mL of vinegar into a cup. Add a piece of chalk into the container and observe what happens. Pour 50ml of vinegar into another cup Teko Mmolawa 5. Soak some steel wool in this cup for 20 minutes. 6. After 20 minutes remove the steel wool and dry it. 7. Let it sit out for about 40 minutes and observe if any rusting occurs Volcano: 1. 2. 3. 4. 5. Place a cup in the middle of the container. Pour 50mL of vinegar into the cup. Add 10mL of detergent and a few drops of food coloring. Tape the funnel over the cup and color and decorate as desired! Add 10g of baking soda and observe the eruption Try to blow up a balloon with the gas that is given off. Experiment with different amount of vinegar and baking soda, to get slow volcanoes and fast volcanoes. Students could also use lemon juice and soda instead of vinegar to learn that different substances are also acidic. Source: 1. Acid Rain (accessed 21st January 2012): http://en.wikipedia.org/wiki/Acid_rain 2. What are the effects of acid rain? (accessed 21st January 2012) http://www.letstalkscience.ca/index.php?option=com_sobi2&sobi2Task=sobi2Details&c atid=4&sobi2Id=107&Itemid=43 3. How can I make a volcano? http://www.letstalkscience.ca/index.php?option=com_sobi2&sobi2Task=sobi2Details&c atid=4&sobi2Id=104&Itemid=43 Teko Mmolawa Name__________________________ Acid Rain! Paul Mevanne and Henry Whatisse have been rival sculptors since their childhoods. Mevanne is known for making incredible sculptures out of plastic and glass, while Whatisse loves using marble, and metals. For the past 6 months they have both been hard at work creating sculptures for the annual art competition. Both artists love working outdoors so the public can enjoy their art Two nights ago it rained heavily. The following day most of Whatisse’s sculptures were destroyed. Mevanne’s artworks were not affected by the rain at all. Whatisse accused Mevanne of destroying his works the few days before the art competition, but Mevanne denies it. What could have happened to Whatisse’s sculptures? 1. 2. 3. 4. 5. Pour 50ml of vinegar into 2 separate cups. Add the small pieces of chalk into one cup and observe Soak steel wool in the other cup for 20 minutes. Remove the steel wool and dry it out with a paper towel Let it sit out for about 40 minutes and observe what happens 1. Chalk: What changes did you see taking place? 2. Steel Wool What happened to the steel wool after soaking in vinegar and letting it sit out for some time? Teko Mmolawa Volcanoes! 1. 2. 3. 4. 5. Place a cup in the middle of the container. Pour 50mL of vinegar into the cup. Add 10mL of detergent and a few drops of food coloring. Tape the funnel over the cup and color and decorate as desired! Add 10g of baking soda and observe the eruption Try to blow up a balloon with the gas that is given off. Experiment with different amount of vinegar and baking soda, to get slow volcanoes and fast volcanoes. Questions: 1. How could you make a fast reacting volcano? 2. How could you make a slow reacting volcano?