What is the formula mass of C 2 H 5 OH?
advertisement

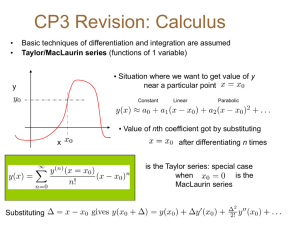
QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. Formula Mass & Molecular Mass Formula Mass Formula mass is the total of all the atomic masses in a chemical formula. Formula Mass & Molecular Mass Every molecular mass is a formula mass... Formula Mass & Molecular Mass but not every formula mass is a molecular mass. Formula Mass & Molecular Mass Can you explain why? Formula Mass & Molecular Mass Are molecules the only things that have chemical formulas? What is the formula mass of C2H5OH? What was the definition of a formula mass? The total of all the atomic masses in a chemical formula. The total of all the atomic masses in a chemical formula. C2H5OH QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. X2 X6 X1 The units of formula mass are: Atomic Mass Units Quic kT i me™ and a T IFF (Unc ompres s ed) dec ompres s or are needed t o s ee thi s pi c ture. amu or u Formula Mass Calculation C2H5OH C = 12 X 2 = 24 How to do H= 1X6 = 6 formula O = 16 X 1 =mass 16 calculations. 46 u Formula Mass Calculation C2H5OH C = 12 Write X 2 = 24the H = 1 Xchemical 6 = 6 O = 16 X 1 = 16 symbols 46 u like this. Formula Mass Calculation C2H5OH C = 12 XWrite 2 = 24 the H = 1 Xatomic 6 = 6 O = 16 X 1 = 16 mass of 46 u each. Formula Mass Calculation C2H5OH C = 12 X 2 Multiply = 24 H = 1 X 6 by = 6the O = 16 X 1 subscript = 16 of46 each. u Formula Mass Calculation C2H5OH C = 12 X 2 = 24 H= 1X6 = 6 O = 16 X 1 = 16 46 u Equals Formula Mass Calculation C2H5OH C = 12 X 2 = 24 Circle your answer. H= 1X6 = 6 Show the units. O = 16 X 1 = 16 46 u Formula Mass Calculation C2H5OH C = 12 X 2 = 24 H= 1X6 = 6 O = 16 X 1 = 16 46 u Formula Mass Calculation +2 SO4 S = 32 X 1 = 32 O = 16 X 4 = 64 96 u Formula Mass Calculation +2 SO4 S = 32 X 1 = 32 O = 16 X 4 = 64 96 u Practice Calculating Formula Mass What is the formula mass of iron (II) sulfate? FeSO4 FeSO4 Fe S O FeSO4 Fe = 56 S = 32 O = 16 FeSO4 Fe = 56 X 1 S = 32 X 1 O = 16 X 4 FeSO4 Fe = 56 X 1 = 56 S = 32 X 1 = 32 O = 16 X 4 = 64 FeSO4 Fe = 56 X 1 = 56 S = 32 X 1 = 32 O = 16 X 4 = 64 152 u Formula Mass Homework Assignment Hydrated Crystals Crystals that have water molecules chemically bonded to the ions in the crystal. QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. This occurs when a highly ionic substance crystallizes from a water solution. Although these crystals are solids, the water molecules can be forced out of the crystal lattice by heating. CuSO4 5H2O The formula for "hydrated" copper (II) sulfate above indicates there are 5 water molecules for every 1 copper (II) sulfate. CuSO4 5H2O What is the formula mass of copper (II) sulfate pentahydrate HINT: treat water as a separate element in the formula. CuSO4 5H2O Cu = 1 X 64 = 64 S = 1 X 32 = 32 O = 4 X 16 = 64 H2O = 5 X 18 = 90 250 amu How much water (in grams) is in 5 grams of copper (II) sulfate pentahydrate? Quic kTime™ and a TIFF (Unc ompres sed) dec ompres sor are needed to see this pic ture. CuSO4 5H2O looks something like this: QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. Quic kTime™ and a TIFF (Unc ompres sed) dec ompres sor are needed to see this pic ture. Anhydrous CuSO4 looks something like this: QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. Percentage Composition Each element makes up a % of the substance. Percentage Composition Add one step to a formula mass calculation. Percentage Composition What is the percentage composition of C2H5OH? Percentage Composition C2H5OH C = 12 X 2 = 24 H= 1X6= 6 O = 16 X 1 = 16 46 u Percentage Composition C2H5OH C = 12 X 2 = 24 H= 1X6= 6 O = 16 X 1 = 16 46 u Now divide EACH atomic mass by the formula mass. Percentage Composition C2H5OH C = 12 X 2 = 24 / 46 = 52 % C H = 1 X 6 = 6 / 46 = 13 % H O = 16 X 1 = 16 / 46 = 35 % O 46 u Practice Calculating Percentage Composition What is the complete percentage composition of sulfuric acid? H2SO4 H=1X2 = 2 S = 32 X 1 = 32 O = 16 X 4 = 64 98 H2SO4 H = 1 X 2 = 2 /98 S = 32 X 1 = 32 /98 O = 16 X 4 = 64 /98 98 H2SO4 H = 1 X 2 = 2 /98 = 2% H S = 32 X 1 = 32 /98 = 33% S O = 16 X 4 = 64 /98 = 65% O 98 Percentage Composition Homework Assignment Sugar In Bubble Gum