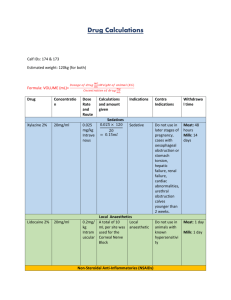
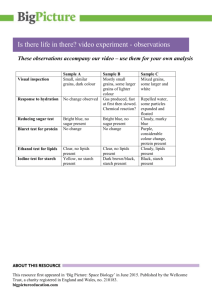
6. Microbiological analysis of agricultural products
advertisement

Quality Analysis of Agricultural Products Sipos, Péter Created by XMLmind XSL-FO Converter. Quality Analysis of Agricultural Products Sipos, Péter TÁMOP-4.1.2.A/1-11/1-2011-0009 University of Debrecen, Service Sciences Methodology Centre Debrecen, 2013. Created by XMLmind XSL-FO Converter. Tartalom Tárgymutató ....................................................................................................................................... 1 Preface ................................................................................................................................................ ii 1. 1. Aims, principles and levels of products grading and quality control .......................................... 3 1. Definition of quality .............................................................................................................. 3 2. Elements of quality control ................................................................................................... 3 3. Sources of analytical methods ............................................................................................... 6 4. Properties of the analytical methods ..................................................................................... 6 5. Practical criterions for the selection of proper analytical method ......................................... 7 2. 2. Principles of sampling of different agricultural products ........................................................... 9 1. Definitions and types of sampling and lots ........................................................................... 9 2. The practices of sampling ................................................................................................... 12 3. Sample preparation .............................................................................................................. 16 3. 3. Sensory and physical quality evaluations. Role and analysis of water content ........................ 18 1. Sensory evaluation of foods and feeds ................................................................................ 18 2. Physical properties of agricultural products and foodstuffs ................................................ 19 3. Role and analysis of water content ...................................................................................... 22 4. 4. Methods for the determination of macro chemical components of agricultural products ........ 26 1. Nitrogen-containing compounds ......................................................................................... 26 2. Lipid content ....................................................................................................................... 28 3. Carbohydrate content ......................................................................................................... 29 4. Ash content ......................................................................................................................... 30 5. 5. Methods for the determination of micro chemical components of agricultural products (mineral elements, vitamins, antioxidants, enzymes, organic and inorganic contaminants) ........................... 32 1. Mineral elements ................................................................................................................. 32 2. Vitamins and provitamins ................................................................................................... 33 3. Antoxidants ......................................................................................................................... 34 4. Enzymes .............................................................................................................................. 34 5. Organic and inorganic contaminants ................................................................................... 35 6. 6. Microbiological analysis of agricultural products .................................................................... 37 1. Sampling for microbiological analysis ................................................................................ 37 2. Methods of microbiological analysis .................................................................................. 37 3. Qualitative determination of microbes ................................................................................ 38 4. Other microbiological methods ........................................................................................... 42 7. 7. Requirements for cereals and their products (Physical, chemical and functional properties) I. 43 1. Quality of wheat .................................................................................................................. 43 2. Physical parameters ............................................................................................................. 43 3. Chemical properties ............................................................................................................ 44 4. Rheological properties ........................................................................................................ 46 5. Other quality parameters ..................................................................................................... 48 6. Qualification of wheat ......................................................................................................... 49 8. 8. Requirements for cereals and their products (Physical, chemical and functional properties) II. 51 1. Quality and qualification of rye .......................................................................................... 51 2. Quality and qualification of corn ........................................................................................ 51 3. Quality and qualification of rice .......................................................................................... 55 4. Quality and qualification of barley ...................................................................................... 56 5. Quality and qualification of oat ........................................................................................... 57 6. Quality and qualification of grain sorghum and millet ....................................................... 58 9. 9. Requirements for industrial crops I. (sugar beet and oil plants) ............................................... 59 1. Chemical composition and qualification of sugar beet ....................................................... 59 2. Chemical composition and quality of oil seeds ................................................................... 61 3. Qualification of oil seeds .................................................................................................... 62 10. 10. Requirements for industrial crops II. (potato and tobacco) ................................................... 65 1. Quality of potato ................................................................................................................. 65 2. Classification and qualification of potato ............................................................................ 66 3. Industrial qualification ........................................................................................................ 67 4. Quality of tobacco ............................................................................................................... 68 iii Created by XMLmind XSL-FO Converter. Quality Analysis of Agricultural Products 5. Qualification of tobacco ...................................................................................................... 69 11. 11. Requirements for fruits and vegetables ................................................................................. 71 1. Chemical composition of fruits and vegetables ................................................................... 71 2. Requirements on fruits and vegetables ................................................................................ 74 3. Ripening of fruits ............................................................................................................... 76 12. 12. Determination of meat quality I. (general considerations) .................................................... 79 1. Post mortem processes in meat and their effects on its quality .......................................... 80 2. Quality and quality analysis of meat .................................................................................. 81 3. Quality of fat ....................................................................................................................... 83 13. 13. Determination of meat quality II. Qualification of bovine, ovine, porcine, poultry and seafood) ........................................................................................................................................................... 85 1. General hygienic parameters of slaughter ........................................................................... 85 2. Sources of quality requirements on meat ............................................................................ 86 3. Qualification of bovine meat ............................................................................................... 87 4. Qualification of porcine meat .............................................................................................. 89 5. Qualification of ovine meat ................................................................................................. 90 6. Classification of poultry meat ............................................................................................. 90 7. Requirements on fish and seafood ...................................................................................... 91 14. 14. Requirements and properties of milk and egg ...................................................................... 92 1. Quality of milk .................................................................................................................... 92 2. Quality of eggs .................................................................................................................... 95 15. 15. Systems for products grading and quality certification laboratories ..................................... 99 16. REFERENCES ......................................................................................................................... 103 17. SAMPLE QUESTIONS ........................................................................................................... 109 iv Created by XMLmind XSL-FO Converter. Az ábrák listája 2.1. Figure 1.: Sampling scheme. ........................................................................................................ 9 2.2. Figure 2.: OC curve for different sample sizes .......................................................................... 11 2.3. Figure 2.: Sampling tools for grains .......................................................................................... 13 2.4. Figure 3.: Decreasing the amount of sample (bisection, quartering) ......................................... 14 2.5. Figure 4.: Bisection of grain sample by sample splitter ............................................................. 14 2.6. Figure 5.: Number of laboratory samples – commercial grain sampling ................................... 15 3.1. Figure 7.: Sorption isotherms .................................................................................................... 22 4.1. Figure 8.: Composition of a food or feed sample ...................................................................... 26 4.2. Figure 9.: Comparison of methods for the determination of nitrogen content ........................... 27 6.1. Figure 10.: Conventional microbiological analysis of foods (Feng, 2007) ................................ 38 7.1. Figure 11.: Representative Farinograph diagram ....................................................................... 47 7.2. Figure 12.: Representative Alveograph diagram ....................................................................... 47 7.3. Figure 13.: Representative Extensigraph diagram ..................................................................... 48 9.1. Figure 14.: Parts of sugarbeet root and their sugar content ........................................................ 59 11.1. Figure 15.: Change of respiration rate of climacteric and non-climacteric fruits .................... 77 12.1. Figure 16.: Structure of muscle ................................................................................................ 79 12.2. Figure 17.: Post mortem processes in meat (Dióspatonyi I., http://www.chemonet.hu) .......... 81 14.1. Figure 18.: The structure of eggs ............................................................................................. 96 v Created by XMLmind XSL-FO Converter. A táblázatok listája 5.1. Table 1.: Organic and inorganic contaminants of food and forage ............................................ 35 7.1. Table 2.: Detailed quality requirements on wheat (MSZ 6383:1998) ........................................ 49 8.1. Table 3.: Requirements on corn grains for forage (MSZ 12540-1998) ..................................... 52 8.2. Table 4.: Quality requirements for corn for food use (MSZ 6180-80) ...................................... 53 8.3. Table 5.: Quality requirements for sweet corn (Győri-Győriné Mile, 2011) ............................. 53 8.4. Table 6.: Maximum percentage of miscellaneous impurities in paddy rice (EC No 489/2005 of 29 March 2005) ..................................................................................................................................... 55 8.5. Table 7.: Quality requirements for barley for forage use (MSZ 6372-78) ................................. 56 8.6. Table 8.: Quality requirements for malting barley (MSZ-08 1326-79) ..................................... 57 9.1. Table 9.: Classification of plant oils by the main fatty acid (Győri, 1995) ............................... 62 9.2. Table 10.: Chemical composition of different rapeseed variety groups (Lukács P, 1987 in Győri, 1995) ................................................................................................................................................. 63 11.1. Table 11.: Chemical composition of main vegetables (Győri, 1999) ...................................... 72 11.2. Table 12.: Chemical composition of main fruits (Győri, 1999) ............................................... 73 12.1. Table 13.: Chemical composition of different meat kinds (Heinz and Hautzinger, 2007) ...... 84 13.1. Table 14.:Minimum requirements on meat quality (UNECE standards: ECE/TRADE/326, ECE/TRADE/308, ECE/TRADE/369, ECE/TRADE/358, ECE/TRADE/355) ............................... 86 13.2. Table 15.: SEUROP classification criteria for bovine by the development of carcass (EC No. 8/1994; EC No 1249/2008; Ostojić-Andrić et al., 2012) ............................................................................... 88 13.3. Table 16.: SEUROP classification criteria for bovine by fat cover (EC No. 8/1994; EC No 1249/2008; Ostojić-Andrić et al., 2012) ........................................................................................... 88 13.4. Table 17.:SEUROP classification criteria for porcine (EC No 1234/2007 Commission Regulation; Decree No. 136 of 2011 (XII.22.) of the Ministry of Rural Development) ...................................... 90 14.1. Table 18.: Chemical composition of milk ................................................................................ 93 14.2. Table 19.: Physical and chemical requirements on the raw cattle milk (Codex Alimentarius Hungaricus 2-51) .............................................................................................................................. 93 14.3. Table 20.: Biological requirements on the raw cattle milk (Codex Alimentarius Hungaricus 2-51) ........................................................................................................................................................... 94 14.4. Table 21.: Requirememts on the pasteurizated milk products (Codex Alimentarius Hungaricus 2-51) ........................................................................................................................................................... 94 14.5. Table 22.: Chemical composition of eggs (Tanács, 2005) ....................................................... 96 vi Created by XMLmind XSL-FO Converter. Tárgymutató 1 Created by XMLmind XSL-FO Converter. Preface The subject Quality analysis of agricultural products for Agricultural engineer MSc students is a complex one. Its general purposes are to improve the student’s competence for understanding the importance of different quality parameters in agricultural or food use and preparing them for the interpretation of the process and results of quality control. To fit this aim, several theoretical, legal, chemical and analytical fundamentals have to be owned as this subject has no opportunity to describe them in details. Therefore, the structure of this subject can be separated into four parts. The first part presents the general issues of quality control; definitions, aims and principles. The second part summarizes the possibilities of physical, chemical and microbiological analysis used in the quality control of agricultural products, the principles of main methods used in quality analysis. The third part presents the quality requirements of agricultural products, focusing on the role and effects of different parameters on the quality of end-product. The last part familiarize with the different kinds of analytical laboratories; the levels used for their certification and its details. Because it is a very wide knowledge scope, this teaching material cannot be a comprehensive one either in the analytical methods or in the detailed product groups, but it try to establish an approach, what helps to use and expand the acquired knowledge to different fields. ii Created by XMLmind XSL-FO Converter. 1. fejezet - 1. Aims, principles and levels of products grading and quality control 1. Definition of quality Quality is a simply and a complex property of an object at the same time. Simply, because a single people or consumers have demands on the object of the analysis and can make decision on its value or usability and can easily classify the products by their quality. However, in the official or trade classification there are no levels for quality, it means only the suitability of a product. As the ISO 9000:2000 says, quality is „the degree to which a set of inherent characteristics fulfils requirements”. The MSZ EN ISO 8402:1994 Hungarian standard gives a similar definition: quality is a set of characteristics of an unit which influence its ability to fulfil specified and exacted demands. Requirements can be pronounced demand or expectation, obligatory or axiomatic ones. The complexity of quality in the case of foodstuffs or agricultural product can be followed by the complexity of the requirements. The basic demands are the safety, consumption value, nutritional value, suitability and other consumer’s demands. The feed or foodstuff have to be safe to consume, that is to say it can not cause any harm to the consumer’s health. In general it summarizes those set of criterions, which provide that the food or feed will not be harmful to health if the consumer consumes it. This is a pronounced, obligatory demand, and however axiomatically is it, several regulations deal with this question. If the product is not safe it is not suitable for consumption however high its nutritional value is. The second level of the complex quality approach is the suitability – is the product meets the requirements of the specific processing or fresh consumption? The third one is the nutritional value – if the product suitable for use, what is its nutritional value, thus in what capacity and amount can it meet the consumer’s physiological demands? The fourth is the consumption value – if the product is safe and suitable to use and has a specific nutritional value, will the consumer accept or prefer it with its other properties? It means meanly sensory properties: those parameters of product which will be experienced by the consumer during consumption The fifth group based the customer’s individual expectations and demands. For example, different sensory and nutritional properties of foodstuff mean better quality for an infant or elderly. On the other hand, personal characteristic is the applied diet (for example, the vegetarian or paleolithic diet). Huge role has the practice and traditions of meal in the family - is it done usually together or just does everyone eat something when needed? The ethical and religion based expectations (e.g. halal or kosher food) are also very important both for the costumers and the quality analysis. And finally, other factors are also influence the acceptance of a foodstuff; package, storability, volume, association with previous expectations or experiences. The question if suitability is also a complex one. In general it means that the product is suitable for a specific use. However, the elements of suitability are quite different in the different levels of the processing chain. In the agriculture quality means different concepts on the different levels. For the producer the good quality can be experienced with yield, high and rapid mass growth, resistance to biotic and abiotic stress factors, homogeneity, good and long-term storability and, after all, a marketable product. For the processor, a raw material with good quality means all those properties which provide an easy and quality processing and end-properties for its product, for example it is homogeneous, its physical, chemical and other properties make it suitable to be worked good and easily, its use will result low amount of losses during processing. The merchant is interested on the marketability of the product; will it be attractive for the consumer, will its package and labelling arouse the consumer’s interest, is it storable for the required period? And, after all the consumer’s demands are the safety of the product and the high consumption and nutritional value. At the same time, other specific demands are also present; labelling has to be pleasant, the processing level be as low as it is possible – or on the contrary, be ready-to-use, contain as little number of artificial components as it is possible, be able for long-term storage, and so on. Kent (1975) collected quite clearly the differences in the requirements on the different levels of production of wheat. He found that the producer requires good growing properties, high yield while it cares for quality parameters if it means differences in price. The miller requires good milling quality, favourable storability, higher flour yield. The baker requires suitable flour for bread, paste or biscuit making with constant quality, while the consumer requires a tasty, healthy product for reasonable price with attractive appearance. 2. Elements of quality control 3 Created by XMLmind XSL-FO Converter. 1. Aims, principles and levels of products grading and quality control Quality control is the process when the quality of a product is investigated, thus, the process of decision making on the suitability or acceptance for the specific use. Its general steps are the sampling, what will result a sample to control; the sample preparation, when the amount of sample is reduced and it made ready for the direct analysis, the analytical measurement, when the direct analysis is made and the conclusion drawing, which includes the data processing, the result preparation and verification. All the elements of this chain have to be work properly, because the weakest ulink will determine the accuracy of the quality control process. Significant amount of total errors caused by the inadequate sampling, references say it causes more than 80% of total error of analysis. The sample preparation is done in laboratory conditions, the special knowledge of the stuff reduces the possibility of mistakes; 8 to 15% of the analytical errors are from this stage. The measurement systems are partially or fully automatized systems, so the error of their work is above 5% in general. The conclusion drawing is the black-box of the quality control; if it works properly, it will not cause inadequacy, but the lack of expertise may cause also significant error on the result. In general, drawing the right conclusion requires competent human resources and adequate circumstances (including equipment, facilities, methods, background activities). It is not surprise that the aim of the laboratory accreditation is the validation of these factors. For the right conclusion making the access of information is very important; what is the aim of the control, what is the material have to be analyzed, what is the purpose of the use of the product? For the proper results, representative sampling have to be applied which fits for the material and purpose. For example, different sampling method and tools are required when the sample is taken from wheat or soil. Similarly, the handling and storage of samples are different when the feed quality or the microbiological status of a corn lot has to be controlled. Similarly, the method of sample preparation and measurement also has to be adoptable for the purpose; for example an extractant does not destruct an important compound of the sample matrix. If any factor of sampling or sample preparation may influence the decision making it have to be considered, so the elements of this system have to fit and cross-ulinked. As many aspects of quality are available, many demands against the quality control are present. The first one is to determine if the product is suitable for human or animal consumption. The second one is the knowledge of nutritional or feeding value; the third one is determination of the technologically important quality parameters. In several cases these demands are present simultaneously; a quality parameter may determine the nutritional value to a certain degree and influence the industrial use, for example protein content of winter wheat. Another question is that if it is necessary to know the exact amount of components or is it enough to know the presence? What kind of detection limit is necessary to meet the purpose? And finally, the requirements change by time – these changes have to be followed both in the scope of parameters and in the detection limits. The quality control has two main parts: the determination of a value for a parameter characterizes the sample and deciding whether the sample does or does not meet the requirements? For the second part limit values have to be considered for the different quality groups. These limit values can be classified by: • scope • mandatory standards • voluntary standards • source Mandatory standards are the ones which have to be fulfilled by all the products which will be used for the specific purpose. It contains safety-related parameters (for example permitted levels of contaminants) and other ones (e.g. nutritional parameters as protein content or physical parameters as impurities) also. Mandatory standards can include requirements on identity, quality and others. The demand on identity defines the parameters which make a product to a specific one; its raw materials or the way of processing. For example if a milk product is made from cattle milk it is enough to indicate “milk”, but if it made with the milk of other mammal, the species of it and the amount of presence also have to be shown The fresh attribute for a milk product can be used when the pasteurization was performed on 72°C for 15 s. The demands on quality are regulations on the physical (e.g. mass, maturity, impurities), chemical (e.g. moisture content, sodium chloride content), biological (e.g. maturity) or microbiological (e.g. mould count) parameters. The other mandatory parameters are ulinked to the package, filling of the container, storage and other production parameters. The voluntary standards can be applied by bilateral agreements of the seller and buyer, or give the base the grading of the products (extra, first, second and other classes). This means that a product has to meet the requirements of the mandatory standards to use for the specific purpose, but its value (price, acceptance) may be modified by the voluntary standards. 4 Created by XMLmind XSL-FO Converter. 1. Aims, principles and levels of products grading and quality control The source of quality standards can be national or international one. To distribute a product in a country the national standards have to be considered, but these ones often harmonized with the international ones. In the international trading international standards have to be applied. General rule is the requirements of a lower level standard cannot override the requirements of a higher level one. The most frequent sources of standards for product quality are the United Nations Economic Commission for Europe standards, the regulations of Codex Alimentarius and the regulations of the European Commission in the European Union. These and the national standards can be complemented by other standards, for example recommendations of an international or national organization or by industrial demands and limit values. The United Nations Economic Commission for Europe (UNECE) standards are developed and revised by the Working Party on Agricultural Quality Standards (WP-7) of UNECE. Their agricultural standards aim to help the international trade. These regulations are used by the governments, producers, traders and other participants of the chain in the countries of United Nations. They cover a wide range of agricultural products containing detailed requirements and recommendations for: • Fresh fruit and vegetables (FFV) • Dry and dried products • Seed potato • Meat • Eggs • Cut flovers Because of the increasing concern about food safety and quality, the Food and Agriculture Organization of the United Nations (FAO) and the World Health Organization (WHO) founded the Codex Alimentarius Committee in the 1960ies. Its declared aim is to manage and help the working out of requirements for foods, to organize internationally the harmonization of Codex Alimentarius and to help trade processes. The Codex contains detailed horizontal issues, which have to be applied on all the food products and vertical issues, which deals with a specific food groups. The structure of the Codex: • general requirements on food hygiene and residues of pesticides and veterinary drugs • requirements on foods for special dietary uses • requirements on product groups (processed and quick-.frozen goods, fresh fruits and vegetables, fruit juice, cereals and pulses and their derived products, vegetable proteins, fats and oils and related products, fish and fishery products, meat and meat products; soups and broths, sugars, cocoa products, chocolate, confectionery products, milk and milk products • accepted methods of sampling and analysis The joined countries establish their national working committees (in Hungary this is the Hungarian National Committee of FAO / WHO Codex Alimentarius), which organize the general work related to the Codex by the Codex Working Committees established for the specific activities and fields (e.g. food hygiene, sugar, processed fruits and vegetables). The Hungarian National Committee is financed by the Ministry of Rural Development. The national Codex regulations contain all the mandatory requirements which are necessary to meet for placing a product on the market. The legislations of European Commission and their harmonization are containing general and specific requirements for the food products. Their aim is partially common with the previous ones: the protection of consumers and the furtherance of economic progress. The first of these aims both contains regulations on hygiene and contaminants, but administrative consumer protection issues are also included (e.g. on labelling, packaging). The furtherance of economic progress is helped by the Common Agricultural Policy and other legislations fostering the purity of competence on the market and the regulation of export and import. The legislations accepted by the European Commission have to be harmonized and also accepted by the member countries in national laws and other legislations. In Hungary the highest level food related law is the Act No. XLVI of 2008 on food chain and its control, which regulates the control of the food chain and the liabilities and responsibilities for the private and governmental participants, contains the rules for distribution and advertising 5 Created by XMLmind XSL-FO Converter. 1. Aims, principles and levels of products grading and quality control of foods, the ways of official procedures, provides the tools of traceability in the food chain, collects the professional mandatory requirements and recommended guidelines (in this, Codex Alimentarius Hungaricus) and formulate the basic definitions. 3. Sources of analytical methods To perform the quality control appropriate method for the sampling and analysis is required. There are several sources for them. If the purpose of the control is data providing for official use (licensing, official inspection) standardized methods or other methods accepted by the national legislation have to be chosen. In Hungary the previously presented sources are the official ones. Validated and certified methods of accredited laboratories are also can be accepted. If the purpose is the determination of price between the commercial partners, other methods named in the contract can be used. Several methods of different associations and organizations are available (e.g. Association of the Official Analytical Chemists – AOAC, American Oil Chemists Society – AOCS, International Association for Cereal Science and Technology – ICC) and they also can be accredited. The equipment and reagent suppliers are also provide ready-to-use analytical applications from the laboratory to the industrial and informative scale. The results of the newest method developments can be found and became available from scientific articles and today the internet is also a useful tool in the method searching. The quality control can be classified by the type of quality parameter want to be determined. In general, physical, chemical and biological (microbiological) groups of methods can be separated, but by the purpose of the analysis there are other options for grouping: sensory, rheological, nutritional and food safety. Sensory parameters also includes physical, chemical and microbiological properties, rheological parameters are influenced by physical and chemical characteristics, while both nutritional and food safety properties are influenced by the general groups of quality parameters. Similarly, the importance, judgement and the desirable level of a specific component are strongly influenced by the use of material and the purpose of control. The high protein content is favourable for winter wheat when it is used it in baking industry but disadvantageous in the brewery industry – but the method for determination is the same. The high amount of gluten proteins is required for pasta industry, but its presence is undesirable in foodstuffs which may available for peoples suffering in celiac disease – the purpose and possible methods of determination are different. A simple, cheap and costeffective washing method is needed to determinate the gluten content for pasta making, but sensitive, professional demanding chemical or biological method is required to detect the presence of the undesired component, the gluten in the foods. 4. Properties of the analytical methods To choose the analytical method the most appropriate method has to be selected. The different methods have different operational parameters and demands, but the users of the results are also worth to know the reliability of analysis. The characteristics of the methods are the following: • Accuracy: the difference between the real value and the measured one. The real value is very rarely known; reference materials can help to determine the precision of a method. • Precision (or reproducibility): the difference between two or more measurements on the same sample. The real value is not known • Simplicity of operation: how easy to perform the measurement; simply theorem and implementation vs. sophisticated background and difficult implementing. • Cost: the cost of a method can be calculated from the acquisition cost and amortization, the cost of consumables, reagents, chemicals and the working hours of technicians and engineers. • Speed: the time of measurement from the starting of the analytical method to the receipt of measured signal. The time of sample preparation may significantly modify the assessing of this operating parameter. • Sensitivity: the smallest measureable difference between the responses of the analytical method. With other words: the smallest difference the method can observe. • Specificity (or selectivity): the specific component to what degree can be separated from the sample matrix. The more specific method less sensitive to disturbing factors. 6 Created by XMLmind XSL-FO Converter. 1. Aims, principles and levels of products grading and quality control • Safety: how dangerous is the implementation of measurement. The used chemicals, instruments and tools will determine the level of danger. • Destructive/Nondestructive: non-destructive methods are more preferred if the amount of sample is low or only small quantities of examined material are available. It is typical for samples from experiments and breeding activity. The non-destructive methods have other benefits typically; lower chemical needs (environmental aspects) and smaller time to perform. • On-line/Off-line: the measurement can be installed into or on the processing line. Online methods are more favourable for industrial practice and automatized processing lines, but in general they have lower sensitivity, specificity and accuracy, higher investment costs, but easy and more cost-effective to use. • Officially approved: when the result will be used in official business, standardized or approved method is required to use. • Nature of sample matrix: in what conditions can be the method used? What are the specific parameters of the sample (as the environment of the detected component) which will provide suitable conditions for the accurate determination? • Tolerance to disturbing factors (robustness, stability, asset and environmental resistance): to what degree will the signal (the result of the measurement) change by the changes of the environment? When a method is adopted the original operating characteristics are also be kept? • Uncertainty of measurement: statistically predicted parameter for the measurement. The results will always be affected by errors, but the repetition of a measurement by infinite times will decrease the error to zero. In the practice there is no opportunity to make as many repetitions which will decrease the error level below the desired level, so the validation of the method include a prediction to the possible error level. There are several methods available to measure a specific parameter in several cases, but the knowledge of characteristics listed above can help to choose or exclude ones from the possibilities. For example, protein content can be determined by several methods; Kjeldahl method, Dumas-method, Barnstein method, spectrophotometric method and so on. But some of these methods measure only the nitrogen content, and the form of the nitrogen in the compounds is ignored – organic, inorganic, bonded in ammonia, nitrate, or amino acids, amides, proteins. When the aim of analysis is the determination of protein content which can be utilized in the feed for ruminants and the sample matrix contains only a negligible amount of nitrate and nitrite, both the Kjeldahl and Dumas methods for the determination of nitrogen content are suitable, but the high amount of oxidized inorganic nitrogen will cause significant difference between the results. On the other hand, spectrophotometric methods are generally simple and relatively rapid ones, but the colour components of the sample matrix may limit the range of samples can be examined, or the reagent which causes colour reaction reacts only with a part of present proteins (e.g. the ones with –SH groups). 5. Practical criterions for the selection of proper analytical method Will the analysis be performed on field, factory or in a well-equipped laboratory? When the test will be used in the reception of a storehouse, for example a cereal intervention warehouse it is unnecessary to invest and maintain a highly accurate method requires high profession. In contrast, the method with low accuracy is not efficient in an analytical laboratory, because its precision is not meet the high expectations. But if more methods are available and their accuracies are different in different concentration ranges, then a rapid but not so accurate method may help to select the most proper one. (for example determination of protein content by near infrared spectroscopy, spectroscopy and Kjeldahl method) Single or continuous analysis is planned. When the analysis is repeated in time then the concentration range and limits are known and more specialized method can be selected or developed. When the aim of determination is the evaluation of a single state of solid, fluid or gaseous sample a more longer-lasting and accurate method can be selected, but if the monitoring of a continuous flow of material is the purpose of analysis (e.g. the control of a feeder in an automatized processing line) the speed of test is much more important. What is the amount of the available sample? If there is only a very limited amount of sample, then its mass or volume will limit the possibilities. 7 Created by XMLmind XSL-FO Converter. 1. Aims, principles and levels of products grading and quality control One or more component should be analysed. When several components have to be analysed by similar method and the number of samples high and continuous, it is worth to select a more costly but more rapid method (for example mineral element composition can be determined by atomic absorption spectroscopy and inductively coupled plasma optical emission spectrometry with different costs, speed and possibilities of simultaneous.) How much is the detectable component in the sample? Is it a main or concomitant component? The determination of components with high concentrations is possible by more simple, automatized and costeffective methods in general while the ones with low or very low concentrations may require time-consuming and expensive sample preparation. What is the required accuracy? In general, higher accuracy requires higher profession, higher equipment, more strict complying with the optimal operating conditions, more time and chemical use How much time we have for analysis? When the time available for the analysis is short the time-consuming methods are automatically excluded. What kind of equipment do we have? When the laboratory has have different facilities and equipment already, a method applicable on the available equipment is a cost-efficient selection way. The price of analysis. After all, the cost and benefit relations are one of the most important criterions for the method selection. But it is not sure that the cheaper method with the same performance characteristics is the best choice because newer investigation aims emerge in a laboratory from time to time. If a method is chosen and an equipment is purchased, its expandability in the future is also have to be considered. Looking at all the issues and concerns it can be conclude that although the asked questions are different, but the aspects are grouping around only some criterions: accuracy, time, cost, expandability, functionality, specificity. The decision making requires profession and information. From the aspects of the practice (a factory, warehouse reception) the main criterions is the measurement have to be more rapid and objective, reproducible and comparable to reference methods or previous data with reasonable operational costs. In a laboratory where the reliability of results, the concentrations of nutritionally important or contaminating compounds are the most important issues, the accuracy, detection limits and the number of measurable components are also important while the cost and operational time have only secondary importance. 8 Created by XMLmind XSL-FO Converter. 2. fejezet - 2. Principles of sampling of different agricultural products 1. Definitions and types of sampling and lots During the analysis performed in the quality control only a part of the original object or material is evaluated. The evaluation of the whole original material would assure the highest level of accuracy, but in the practice the analysis is not the aim of production, but it is only an avoidless element of the flow of materials. Large amount of products are present in the warehouse, truck, storehouse, factory or diary, but the analysis requires only a small portion of it, for example 1 g of sample for the determination of protein content. Sampling means all those operations which are necessary to make a sample. The collected sample or later the analytical sample is a small (very often very small) part of the evaluated object, it is made by specific methods to provide a representative part for analysis. The sampling has the same purpose than the analysis or a tool makes the analysis possible. It is present in the industrial, commercial, laboratory and informal quality control too and it may be basis for the quality control of raw materials, semi-finished products or the processing line and the final product, to determinate the price or the optimal use of the material, it may help monitoring the changes during storage or testing a method, an instrument or the effect of a treatment. The different purposes may require different kinds of sampling. Taking service and certified samples requires formal sampling by standards, while informative sampling or sampling for the evaluation of an experiment needs the sampling fits best for the purpose. For the discussion of sampling the following definitions are necessary to know: Lot: “A quantity of a food material delivered at one time and known, or presumed, by the sampling officer to have uniform characteristics such as origin, producer, variety, packer, type of packing, markings, consignor, etc.” (Codex Alimentarius) This means that the base of the sampling process is presumably homogeneous and the sampling person have to make certain of there is no sensory or other appreciable difference in the parts or elements of the object to analyse. If the lot is not homogeneous it has to be divided to homogeneous ones. The lots can be classified by their sizes, distribution and homogeneity. The size of lot can be finite, e.g. a stored mass of cereal grains in the warehouse, or infinite, e.g. the same corn sample during storage or milk continuously flowing in the diary. Their distribution can be continuous or compartmentalized, for example the cereal grains in a silo with known volume or stored in sacks. They can be homogeneous or heterogeneous by their origin or other properties; for example the content of a cereal grain silo may be found homogeneous in which all the products are from the same parcel and heterogeneous when the grains are mixed from different parcels, farmers, varieties, agricultural conditions and so on. In fact the homogeneity of a lot containing agricultural products is never full, due to the micro-heterogeneities of the production area and conditions. This is why one sample is never enough to take from a lot which homogeneous from the first sight and the obviously not homogeneous lots have to be divided to parts. Primary sample: “One or more units taken from one position in a lot.” (Codex Alimentarius). The required number of primary samples depends on size of the lot and it can be found in the relevant standard, method or sampling plan Bulk sample: “For products other than meat and poultry, the combined and well mixed aggregate of the primary samples taken from a lot. For meat and poultry, the primary sample is considered to be equivalent to the bulk sample.” (Codex Alimentarius) Bulking provides that the primary samples will form a larger homogeneous one, as the non-appreciable differences make inhomogeneous the sum of primary samples may cause significant error in analysis. In the case of meat and poultry this mixing is not possible due to the physical characteristics of the materials. (Figure 1). 2.1. ábra - Figure 1.: Sampling scheme. 9 Created by XMLmind XSL-FO Converter. 2. Principles of sampling of different agricultural products The general types of sampling are the probability and nonprobability ones. The probability sampling is the sampling type where every unit, piece or element of the lot has the same probability to be added to the sample and this probability can be determined. For example, an apple bulk on the track arrives to the factory and the aim of quality control is the determination of overall quality for pricing. An absolutely homogenized sample is needed to avoid the wrong rating, so the selection of the apples to the sample has to be made randomized. The required mass of sample is 20 kg, the mass of cargo is 20 tons, so the probability of the parts of lot is 1 to 1000 (0.1%). The types of probability sampling: • The simple random sampling is the most simple, when the units or parts of the lot are selected randomly and all parts have the same probability to be selected into the sample (in extreme cases, maybe the apples from their neighbourhoods). • The systematic sampling means that sampling is repeated from a specified time to time or per unit from a randomly selected starting point. For example the apples are ordered in a matrix and from the 5th apple in the left upper corner every 1000th apple is selected. As the order of apples are unknown and the starting point is randomly selected the composition of sample will be randomized. • In the case of stratified sampling the lot is randomized but not fully but in terms of one or some specified properties. Its use is recommended if the lot seems inhomogeneous or shows high variability for the evaluated parameter. First, it is necessary to decompose the lot to subgroups (strata) by the analyzed parameter; the similar parts have to get into the same groups. The basis for grouping is a preliminary or – in the case of agricultural products – sensory evaluation. Second, the sampling can be performed randomly from the groups and the ratio of the size of the subsamples from the different groups in the sample is the same than the ratio of their mass or volume to the whole lot. For example, if the apple of contains three kinds with a ratio 0.25:0.30:0.45, then 5, 6 and 9 kg have to selected, respectively. • The cluster sampling is similar to the stratified sampling but the decomposed groups are decomposed towards to subgroups (clusters). For example, the apples is grouped by variety, mass, maturity status and size is a four level clustering. The selection of sample is also made by the presence of groups in the lot. • In the case of composite sampling the previously selected independent samples combined and mixed to each other and form a new sample. For example two or more trucks sampled and the samples from the different vehicles are combined to one The non-probability sampling types do not provide the same probability for all the parts or units to be selected into the sample because it is impossible or undesired. For example, the apple crates will not be unloaded from the truck in the lack of time or to avoid causing mechanical injuries, or only the apples with full red colour will 10 Created by XMLmind XSL-FO Converter. 2. Principles of sampling of different agricultural products be selected because of the aim on analysis and the ones with other colours have no chance to be selected. In statistical words, the probability of selection cannot be determined and the selection rather depends on the attitude and judgement of sampler person than on statistical considerations. The general types of nonprobability sampling: • Judgement sampling means that only the sampler’s discretion will determine what parts of lot will be selected into the sample. The sampler’s professional and experience highly influence the result because the factor what makes this kind nonprobability one is the kind of the person’s individual decisions. For example, the sampler selects the showy ones or the ones seemed to be average. • Convenience or accidental sampling means that the easily accessible parts of the lot will be selected into the sample. In this case the factor what distorts the same probabilities is the spatial location of the parts of lot. • The sampling is restricted when some parts of the lot are inaccessible and therefore it is impossible to sampling it entirely. In comparison this one to the previous one the difference is that in the convenience sampling the sampler decides in what depth he makes the sampling while in restricted sampling he cannot select from a field. • The quota sampling is similar to the stratified sampling, the lot is disported by different properties, but the ratio of the groups to the sample is not formed by their ratios in the total lot, but determined by other considerations. From other consideration, the sampling can be bulk or acceptance sampling. In the case of bulk sampling the parts cannot be distinguished, identified and separated to constant units. In the case of acceptance sampling, a preliminary selection criterion is set and separates the lot into acceptable and not acceptable parts. This separation can be performed by attributes, freely selected characteristics of the lot separate the lot into two parts, where one part meet the criterion and the second one does not. It also can be performed by variables; when the criterion is a quantitative characteristic of the material. For example, acceptance by attribute can consider the presence of an injury or a pathogen on the units and acceptance by variable can make selection by colour, specific nutrient content or size. The main purpose of the selection of right sampling method is to minimize the error which caused by the improper sampling (e.g. inhomogeneity, unrepresentativeness). The sampling error, similarly to the statistical error, can be classified into two kinds. In the case of the type I error the determined parameters therefore the classification of lot is worse than its real properties justify. Type II error means the situation when the determined value of the lot, its quality is better than the determined. Practically, in the case of Type I error the value of the sample is underestimated and the quality analysis rejects it while it fits the requirements. In the case of Type II error the quality of sample is overestimated, thus it will be accepted although it has worse quality parameters than the required. The probability of the Type I error is α and the probability of Type II error is β (the marks and the background is similar than in statistics). With other words, α is the probability of rejection of an inappropriate lot, while β is the probability of accepting an inappropriate lot. If the qualification requires a 2% level for a specified parameter at most (quality level; p0) and the experienced quality level (p) is lower than 2% (p≤ p0) then the lot will be accepted and if p> p 0 then it will be rejected. Due to the uncertainty of sampling and analysis 2 quality levels is used practically; p 1 is the accepted quality level and p2 is the least quality and α is the risk of the deliver and β is the risk of the recipient. Based on the p 1, p2, α and β operating characteristics (OC) curves can be drawn to characterize connection amongst the ratio of inappropriate products in the lot, the probability of acceptance and the size of sample. It can be seen that the sample size significantly influences both the probability of acceptance and the occurrence of inadequacies in the accepted lot. For example (Figure 2.) a lot will be accepted if it contains 1 or less defective items or %, there is about 58% the probability for accepting a lot contains 8% defective items when we judge on the results of 6 sample and this value decreases to 20% when the sample size increases to 18. 2.2. ábra - Figure 2.: OC curve for different sample sizes 11 Created by XMLmind XSL-FO Converter. 2. Principles of sampling of different agricultural products 2. The practices of sampling The process of sampling has 7 main steps: • Creating a sampling plan or find a relevant standard or recommendation There are several sampling standards and recommendations are available. Both international (ISO International Standard Organization) and national standards are available for the different materials and purposes. Similarly to the selection of methods the purpose of analysis has to be considered during the selection – international transactions require internationally accepted sampling methods. The Codex Committee also has a working committee on the development of methods for sampling (and analysis) aiming to develop, adopt and revise the principles for the use of sampling in international food trade. The standardized and recommended methods developed for specific purposes and sample types – for exampleISO 707:2008 lays down the basics for sampling of milk and milk products or (EC) No 333/2007 (of 28 March 2007) deals with the sampling of foodstuffs for the determination of different inorganic and organic contaminants. When standard or recommendations are no available for the sampling, sampling plan has to be worked out. It considers the purpose of analysis (acceptance or grading; overall quality or contamination from a point source, homogeneity test), the nature of material (physical, chemical and biological properties and homogeneity), the analytical method (size, cleanliness, influence of post-contamination and handling) and the lot (size, homogeneity, environmental conditions, placing and others). It may also specify preliminary (prompt) preliminary examinations (for example sampling has to be rejected when a specified contamination or defect is present). • Collecting the sample When the sampling plan requires preliminary examinations they have to precede the sampling. For example preliminary requirements are the lack of cereal insect pests in the lot, the lot has to be free from mildew and foreign odor, etc. thus a preliminary evaluation is necessary to perform to check these parameters. 12 Created by XMLmind XSL-FO Converter. 2. Principles of sampling of different agricultural products The method for sample collection is written in the standards or sampling plan in details. The sampling requires proper sampling tools. These tools have to fit to the physical characteristics of the lot and to the properties of storage conditions. Different sampling tools are required for solid, liquid and gaseous materials and for the stable and flowing materials. For grains test probes with different length are the suitable tool to sampling; a bulk lot in a warehouse has to be sampled with a probe what length is not less by more than 50 cm than the thickness of lot. This is why probes with different length and extendable or prolongable ones are available. When the grains are stored in sacks, a bag puncture probe is required to use (Figure 2.). For forage core sampler, shovel, scoop or – at a pinch – hand should be used. For the sampling of meat cutter or boning knife, for the sampling of fat, molasses, other liquids glass or steel tubes or pump sampler are the suitable tools. Some cases no sampling tool is required or its use is prohibited; microbial evaluation of water requires sampling directly from the material container to the sample container. 2.3. ábra - Figure 2.: Sampling tools for grains The first step of sample collection is the determination of the number of samples. The control regulates the minimum number of samples depending on the size of lot. For example, the base for sampling is 500 t for cereal grains except for sorghum where 100 t is the base. This means that one sample can represent a lot with maximum this amount, when the quantity is higher more samples are required to collecting, so three samples are required to collect from a lot with a quantity of 1300 t. The lot should be divided virtually to the appropriate sizes and one sample has to represent a clearly defined part of the lot. When the lot is bulk in a warehouse, it might be said that the 1st sample represents the lot stored in the north, the 2 nd on the middle and the 3rd is the south part of warehouse. This provides the traceability between the results and the lot. In the case of continuous sampling, e.g. placing in warehouse by truck, the basis for sampling is the same, the trucks have to be sampled individually and these primary samples have to be collected until the quantity of placed amount reaches the required one (e.g. 500 t for corn) and then it is necessary to start an another sample. In general, the lots stored in sacks forms a sample basis with lower quantity. The next step is the collection of primary samples. The standards and recommendations contain the necessary amount of them. For example, when corn is sampled from truck during placing, the mass of sample will determine the number of primary samples; 5 samples are required above 15 t, 8 between 15 to 30 t and 11 from 30 to 50 t. From bulk samples the cereal intervention standard of EC requires 8 to 10 kg of primary samples collected from 20 points at least by netted distribution. The Hungarian sampling standard requires taking 5 primary samples from a lot above 20 t and this number increases by one with every 5 tons. This means 41 primary samples have to be taken from a lot with 200 t. For lot in sacks the number of necessary primary samples increases by the number of sacks. It is very important that sampling do not change the properties of the lot. The sampling tool should be suitable to sampling across the whole cross-section of sample and it must be dry and clean, free from remains of previous sampling, what prevents the contamination (or cross-contamination) of the examined material and the collected sample. The sampler or any element of the sampling process do not cause contamination in the material and do not cause inhomogeneity in it. On the other hand, the characteristics of the lot do not change during sampling. For example, corn is very sensitive to the physical effects of manipulation, especially if its moisture content is low enough. When the sampling is done improperly, the test probe is closed too strong then this intervention causes grain breakage and the collected sample will contain significantly higher amount of broken grains as it is characteristic for the original lot. • Mixing the primary samples The collected primary samples have to be mixed. The main purpose of mixing is to form one average sample from the primary ones and homogenize them. The latter one is more important; the collection of primary samples from different points of lot is avoiding the analytical error caused by the inhomogeneity of material. As the amount of mixed primary samples are too high (e.g. 41 primary sample from 200 t corn has a mass of 13 Created by XMLmind XSL-FO Converter. 2. Principles of sampling of different agricultural products about 40 to 50kg), the homogenization and the decreasing the amount of sample is made simultaneously usually and results a bulk sample (or laboratory sample). The bulk sample has to show the same properties as the average of the primary samples. The simplest methods for this step are the bisection and quartering (Figure 3.). The jointed and mixed primary samples have to be dumped on a clean surface (e.g. table, trays) and the a half or a quarter of mixed sample has to be kept and the other one or ones have to be discarded. With the repetition of this process the necessary amount of bulk sample can be reached. Mechanical sample splitters also can be used when their operating characteristics are similar to the one of manual splitting (Figure 4.). 2.4. ábra - Figure 3.: Decreasing the amount of sample (bisection, quartering) 2.5. ábra - Figure 4.: Bisection of grain sample by sample splitter The required amount of bulk sample is determined by the demands of analysis. For the evaluation of wheat or corn grain quality 1 to 1.5 kg is required, for sunflower or poppy seeds 0.5 kg at least, for corn on the cob 10 kg at least. The number of bulk (laboratory) samples is depends on the purpose of analysis. In general 3 or 4 samples are worth to form. One will be analysed in the laboratory, the second one will be preserved by the deliver and the third one by the recipient. If the analysis will be performed in an accredited laboratory a fourth sample is 14 Created by XMLmind XSL-FO Converter. 2. Principles of sampling of different agricultural products required to form, this will be preserved in the laboratory. The preserved samples can be used if one of the participants of the quality control process appeals against the decision to repeat the qualification in an independent laboratory (Figure 5.). 2.6. ábra - Figure 5.: Number of laboratory samples – commercial grain sampling • Packaging the sample The bulk samples have to be packed as soon as it is possible avoiding its contamination or changes in its composition and properties. Containers must be able to ensure that the sample will keep its properties during the necessary time. This time is days only for the laboratory sample but months for the preserved sample. The containers have to protect the sample against any external effect, e.g. vapor, water, impurities, microbes, etc. The type of sample container depends on the properties of material and the purpose of analysis. It can be paper-bags, for example for storing grains, but it is worth to remember that paper bags are vapor permeable ones, thus a part of sample stored in paper bag have to be placed in plastic bag to keep the moisture content of sample until the determination of water content. Plastic bags are also used for storing meal samples, but some kinds of forage samples also can placed into them. For gaseous and liquid samples can be placed into glass tubes and bottles with rubber or plastic stopper, or a screw-cap of metal or plastic. The plastic material like solid fats and butter can be place into a wide mouth jar the easiest. If the purpose of analysis requires special kind of sample container it is written in the sampling plan. For example the determination of violate components requires airtight container or the agricultural samples have to be placed only into glass containers for the analysis of some kind of chemicals, pesticide residues. The sample container must be marked with a code, and if it is large enough, other information (date and place of sampling). If its size makes it impossible an unequivocal mark has to be applied for the later identification. • Preparation a minute A minute of sampling have to contain all the necessary information of sampling. If the sampling plan does not specify otherwise, the minimum information are: • the name of the material, (e.g. sunflower seeds) • the place, amount and type of lot (e.g. steel silo, 1000 t, dried corn) • number of bulk samples (e.g. 2 pcs) 15 Created by XMLmind XSL-FO Converter. 2. Principles of sampling of different agricultural products • condition of the lot (e.g. broken packages, evidence of rodent or insect infestation, debris, microbial activity) • reference to the standard used for sampling or the sampling plan • name of sampler and the attendances (representative of parties) • date and place of sampling • all the information necessary to identify the lot (e.g. the first sample is the first sample taken from the silo or the position in the warehouse – from the north part of lot) • Transport and storage of the sample Transport of sample to the laboratory must be made as soon as it is possible. During transport special storing conditions may be required, for example cooling of samples preserving its microbiologic status or avoiding changes in nutrient contents. The time of sample storage is 3 months for the non-perishable samples, but the ones with rapid deterioration (e.g. intermediate product of fermentation). The storage conditions have to be optimal to minimize the deterioration or similar to the ones in which the lot is stored. Spoilage changes are occurring during storage including physical changes (e.g. water loss or uptake, melting), enzymatic changes (e.g. amylase, protease) and microbial changes. 3. Sample preparation The sample has to be prepared to the analysis. Sometimes it is done just after transported to the laboratory ensuring its storability (e.g. samples with high moisture content) or making possible to reducing its volume (e.g. grinding the straw sample). On the other hand, the analysis may also require preliminary processes what are essential for the test, for example removal of disturbing material, agents or compounds, concentration of compound to be analysed, etc. The sample preparation steps can be classified by the type of sample: gaseous, liquid and solid samples require other methods. The classification is made by the aim of preparation in practice. They can be classified into: • Water removal: Samples with relatively high moisture content have to be dried due to prevent the physiological and microbiological losses and spoilage. The drying temperature has to be select carefully considering to the aim pf analysis; for example 40°C and 60°C drying temperature is gentle enough not to destruct organic compounds, 103°C is suitable for the determination of moisture content (evaporation) and higher temperature for analysis of element content. Evaporation is also used for the concentration of liquid samples then the determinable component is present only in small concentration. • Homogenization: The distribution of evaluated property may inhomogeneous in the sample. It can be mix by different stirrers or mixers, for example in the case of liquid samples (for example milk) and the solid ones with small particle size (for example flour). The homogenization sometimes requires size reduction (cutting, grinding, milling) to provide the distribution of materials homogeneous and enough small particles for the further analytical steps (for example, the different parts of cereal grains or sugar beet have different chemical compositions and physical properties what have to be equalized). It can be done in dry conditions or in liquid phase – when volatile or reactive components are present in the sample matrix, external conditions have to be maintained to preserve the sample. • When the sample contains materials what disturb the analysis separation or cleanup is necessary. The previously mentioned concentration is one way of separation (water and the dissolved compounds) for the liquid samples. Separation also can be performed by particle size: filtration, sedimentation and sieving are the most common methods for this purpose. Another aim is the extraction when a specific extractant (for example water, organic solvent or supercritical fluid) is used for separation (when different compounds are extracted for further analysis) or enrichment (when the aim is the increase of the concentration of the compound to be determine). Modern analytical methods use other kinds of separation methods, for example molecular weight based separation, ion charge based separation, size exclusion separation and others. • In some cases the dilution is necessary to set the amount of analysed compound to the optimal concentration range. 16 Created by XMLmind XSL-FO Converter. 2. Principles of sampling of different agricultural products • The digestion can be physical or chemical aimed to release the components to be analyzed. Physical digestions are, for example, granulation, pulping and cuttering and other methods to destruct the physical structure of cells. Chemical digestion usesdigestion agents; for example in the case of the determination of element concentrations the compounds binding the elements are digested by strong acids. • Derivatization means that the component to be analysed is bind chemically in a specific reaction (for example chelate formation) resulting a new compound or complex what is easy to determine. 17 Created by XMLmind XSL-FO Converter. 3. fejezet - 3. Sensory and physical quality evaluations. Role and analysis of water content Based on the type of analysis, methods of quality control can be classified to four groups; sensory, physical, chemical and microbiologic analyses. 1. Sensory evaluation of foods and feeds The role of sensory parameters of an agricultural product is different. It has significant importance in the qualification of fruits and vegetables due their fresh consumption, but the sensory evaluation of other goods is important, because it can advert to the microbial status, the technological value or the consumption value. The advantage of these test is their cheapness, but trained persons are required to perform them. The most important sensory properties are the following • General purity, presence of foreign matters, seeds and defective parts.. The presence of impurities in a product s visible. Impurities can be useful, worthless or harmful ones. Useful impurities in a winter wheat lot are the rye grains, because their presence improves the baking quality of the flour. Worthless impurities are the grains of other cereals when they are healthy and have the same physiological activities than the main cereal has. If their physiological activities higher, e.g. their respiration activities are higher, they may be starting point for deterioration, so they are harmful foreign matters. The toxic or fungal infected grains are harmful ones also. Besides, the presence of foreign matters makes the general impression of lot worse. • Presence of living pestiferous. The living pests in an agricultural product are disgusting, especially in the freshly consumed ones, but it is also undesired in the industrially processed materials, because their activity causes losses in physical, chemical and nutritional terms, but they may make the microbial status of the lot worse and their presence may make the product unprocessable or inconsumable. • Presence of microbes or their activity. The sensory effects of the microbes are changes in colour, taste, odor and texture of product. The unhealthy effects caused by the activity of microbes may appear sooner than their sensory symptoms. • Colour. The colour of healthy and matured product is specific to it. This property is s one of basic criterion in choosing of consumers several cases, but in product grading only trained people can judge objectively. • Odour. The odor of materials corresponds to their chemical compositions, e.g. the odor of fruits is in relation to their volatile components developed in the last stages of maturity, thus it can be an option to the sensory examination of maturity stage. It may also inform about the activity of microbes. • Stage of maturity. The maturity of some products can be estimated by their colour, odour, size, texture and other sensory parameters by their simultaneous examination. • Ratio of components. The visible distribution of raw materials in a product from mixed raw materials is also refers to its quality, e.g. components in a mixed pickles or ratio of legumes to grass or leaves to stems in hay The steps of sensory evaluation ate the following: Selection of panel. For performing an objective sensory evaluation trained, motivated, balanced and healthy panelist are required who can concentrate to the task in the moment, able to recognize the differences in the required scale and describe the experiences. Several cases untrained panel is better to apply; this can represent the consumers and provides more real information on the acceptance and opinion about the product. The size of panel should be 5 to 10 persons for trained panel, but this number should be much higher for untrained panel, selected people with different gender, age and demands. Creating suitable environmental conditions. The conditions for testing have to provide optimal conditions for the control. The testing and preparation rooms should be separated ensuring that the panelist have no 18 Created by XMLmind XSL-FO Converter. 3. Sensory and physical quality evaluations. Role and analysis of water content preliminary information about the items and not influenced by the preparation (the sight, smell, etc.). The testing room has to be comfortable with optimal temperature, humidity and other external parameters ensuring there are no discomfort or disturbing effects what may distract attention. Booths also can be applied. Choosing the proper test type. Based on the aim of test it can be analytical test, when panelist have to be able to perceive differences in the required degree between the levels of the tested parameter and ranking it on a 5 to 10, or affective tests, when the panelist has to make decision on the acceptance or rejection of product or making a preference order amongst them. Analytical test may aim to the recognition of presence of a compound, quantification of differences or description of the differences by a specific terminology. Obtaining representative samples. Representative samples mean no there are not any differences amongst the production, distribution, labelling, serving and marketing of the samples. This uniformity also has to be observable during the representation and tasting of samples. For several products specific requirements are available for sensory analysis. For example the fresh milk has specific colour, odor, taste and texture. These properties have to be clearly known by the analyst and be able to recognize the non-adequacies. So the milker has to recognize the natural changes of milk (e.g. colostrum, presence of foreign odor and colour, the symptoms of mastitits and others) and be able to difference the variety of cow and herd originated errors and acceptable differences. The sensory analysis meat quality is made by the examination of its colour, water holding capacity and texture and the different meat types (normal, PSE, DFD) can be easily characterized. 2. Physical properties of agricultural products and foodstuffs The quality control has several physical opportunities for the analysis. Some of the physical parameters of the different products are in relation the sensory properties (colour, shape) and other ones refer to the chemical composition (heat and electrical conductivity, refractive index). These methods have simple principles and lower operational costs, they are easy to perform, transportable, can be used on field and do not require professional analysts in general. The optical properties of materials used in quality control are their colour, refractive index and response to excitation. The colour is the electromagnetic radiation of the different materials with a wavelength between 380 and 750 nm, what can be detected visually by humans. It is strongly connecting to the sensory analysis of samples, but while sensory tests, based on the tester’s or panelist’s perception, have subjective issues, the instrumental colour measurement is an objective test. It can be used for grading (apple, green pea), determination of maturity stage (tomato, FFVs), refers to technological properties (meat colour) or deterioration (fungal infection, enzymatic and non-enzymatic browning) and the exposure of material to external factors. The identification of colour can be made in the colour triangle where the colours are characterized by two coordinates and the points of triangle-like shape can be rendered to specific wavelengths, thus the maximum brilliances of single colours. Moving inward in this shape the brilliances decreases and colours became pale ones, and the focal or center point is the zero brilliance – the white colour. In measurement vector quantifies are used there three vector component indicate a colour. One possibility is the hue, chroma and brightness system, where the hue value characterizes the wavelength of colour (e.g. greenish-yellow), the brightness value the bright character from the bright to dark (dark greenish-yellow), and the chroma the degree of brilliance from brilliant to pale (pale dark greenish-yellow). The L-a-b system is most frequently used in the analysis of agricultural products; the a and b are the so-called coordinates of a colour in a plane defined by the green-red and blue-yellow colour axes and the L value is a measure on the third axis perpendicular to the colour plane and characterizes the brightness. The axes are scaled from 0 to 100. Not the visible light can only be used for qualification. The near infrared (NIR) spectroscopy analyses the nonvisible light region (wavelengths form 0.8 to 1000 μm). The NIR spectrometers expose the sample to direct light, the sensitive molecules absorb a part of the radiation and became stimulated or excited. The molecularly absorbed or reflected energy on specific wavelengths generates a specific reaction of bounds, what can be symmetric or asymmetric stretch or scissoring), and it modifies the reflected light. The specific wavelengths refers to the chemical components by the active part of molecule (e.g. –OH or –CH croups), since the changes in the light intensities measured on different wavelengths characterizes the amount of molecules. The NIR spectrometers can be used in reflectance and transmittance method; the first means that the light reflected by the sample is detected (NIR), the latter one means that the transmitted light is detected (NIT). Thereby the adaptability of NIR methods depends on the surface of the material and they requires larger amount of samples 19 Created by XMLmind XSL-FO Converter. 3. Sensory and physical quality evaluations. Role and analysis of water content generally. The spectra of detected light is evaluated statistically; the transformation of these spectras and their analysis by multivariate statistical methods result good prediction for water, protein, fat and other macro nutrient contents, but new applications are suitable for screening tests on microcomponents, e.g. the presence of toxins on cereal grains. The refractive index has been used in analytical chemistry for a long time. The base for this type of analysis is that the speed of light is different in the air and in the materials. In general, the refractive index is the quotient of the speed of light in vacuum and in the material. Based on the incidence angle of ray the material refracts it or reflects internally with some difference in the angles of incidence and reflectance. This difference depends on the chemical composition of the material and as the different materials have specific refractive indices it give basis for the analysis. The Snell’s law says that the ray of light radiates from material 1 to material 2 then where α and β are the angles of incidence and refraction, respectively, n1 and n2 are the refractive indices of the materials, respectively. In s simple polarimeter or refractometer the n1 and α values of the cuvette are known, so the determination of a known chemical component can be performed by the measurement of the angle of refraction, when the concentration of main compound is high enough and there are no present optically active other components in the sample. This principle is used for several kinds of compounds and sample matrix, for example for the determination of water content of honey, dissolved solids in fluids or fatty acids in vegetable and animal fats and oils. The size and shape can also be evaluated by sensory analysis, but objective instrumental methods are also available and as these parameters highly influence the consumer’s attitude of product (fruits and vegetables, egg) the objectivity is very important. On the other hand, size and shape are important in preserving the homogeneity of the materials; for example the components of a mixed feed can be separated either during storage with non-disturbed conditions when the sizes of components are very various. Similarly, particle size distribution is important for the evaluation of the stability of liquid-solid matrixes, e.g. due to the settling of fibers in a fruit juice. It is also important in production, e.g. the peeling losses can be higher when the shape of the object is not smooth enough. The most simple size and size distribution analysis is the sieving. For separation a sieve with specific mess (e.g. 0.25 mm for wheat flour) can be used and for distribution analysis a couple of sieves can be used with decreasing mesh sizes. In this case, the thickness of materials on the mash is important, because differences in densities may cause rearrangement in the sample, thus too think sample coating cause significant error. The sedimentation is commonly used to determine the particle size of solid materials in a liquid, but solid materials can also be evaluated by the terms of Stroke’s law, what says that the speed of falling of particles depends on the diameter of particle and the difference in densities. The sample is placed into a liquid medium, where the difference in densities of the sample and the liquid is different and there are no chemical reactions and sorption take place between them. The homogenized sample-liquid matrix is rested for a specific time, and the volume or thickness of sediment is measured. Its backward method is the air classification, when the particles with lower size rise higher when the same velocity of blown air is applied. It is important to remember that density is also important for both sedimentation and air classification and they are often be used for these purposes in the industry (e.g. separation of light seed in a sunflower lot). The light scattering method is not sensitive to differences in the density of particles. The sample contains particles is passed along a light beam and the particles causes diffraction, refraction and reflection. This cause change in the wavelength distribution of the detected light and this scattering can be used for the determination of particle size. The microscopic image analysis is also an objective, but time consuming method. In contrast, the computer aided image analysis is also objective and more rapid method and it can be automated. The measurement of mass is a simple issue apparently, but as it is present in almost the whole analytical chain it is very important to know this property. Mass can be determined by the force what the sample products due the acceleration of gravity. The measure of gravity is different on the different places of the Earth, keeping this parameter constant between laboratories standards and organizations regulate, supervise and control the uniformity of mass measurement. The atmospheric buoyancy also influence the measured value, so the densities of the medium and the sample, the air pressure, vapor partial pressure and the temperature are also have to be considered. The density is the quotient of mass and volume, thus the unity mass of the material and as it is shown above, influenced by temperature and pressure. The specific gravity is the relative density; the quotient of the sample to a reference material (usually water). The most simply way to the determination of density is the 20 Created by XMLmind XSL-FO Converter. 3. Sensory and physical quality evaluations. Role and analysis of water content measurement of the mass of a known volume of sample. It can be made by pycnometers for liquids and solids with some considerations. A part of sample has to be placed into the pycnometer with known volume and mass, and it have to be filled by reference liquid with known density and volume to fill the gaseous parts (void spaces). After the measurement of mass, the result can be corrected by the parameters of reference liquid. When the quotient of the mass and volume of a solid material is determined, the bulk density is determined, what value is the mass of quotient of the mass and volume of the solid material, including gaseous phase. This parameter is also has importance in quality control, e.g. this is the specific value of cereals. Density is often determined by the measurement of specific gravity; e.g. hydrostatic methods and Mohr-Westphal balance are typical relative methods and tools, when the mass of sample is measured both in air and dipped into a liquid with known density. The density gradient column and hygrometers are tools based on the determination of buoyancy forces of liquids also, but the relative measurement is done by the speed of sedimentation and the depth of submerge of hygrometer scale into the sample, respectively. Density can be used for getting information on the chemical composition of sample, e.g. the density of milk should be between 1.029 and 1.033g/cm3 as lower value indicates water addition. The rheologic properties are also important physical parameters. Rheology evaluates the connection between a force applied on a material and the response of material. With other terms, it is the analysis of the connections between the stress and deformation. The general rheologic behaviors are the viscous, plastic and elastic ones and based on these behaviors the materials can be solid-like of fluid-like practically. Rheometers can be used to analyze all kinds of behaviors, the viscous and plastic behaviors are examined by viscometers, the plastic and elastic behavior by texture analyzer in general. Rheometers or oscillation tests apply an alternating force, this way an alternating stress on the material with stable or changing frequency and/or amplitude and the response of material makes possible to characterize the rhelologic behavior, e.g. if there is no angular misalignment between the stress and deformation, the material is elastic, no internal viscosity makes delay in the response. The viscometers can be grouped into three types; capillary tube, rotational and falling body types. In the case of capillary tube viscometers there is tube in what the material flows across and there is a capillary part in it which slows down the speed of flow and the degree of slowing is proportional to the viscosity of material. In the case of rotational viscometers the analysed material is between a rotating and a stationary body. The rotating body indicates a flow within the material and the speed of this flow will decrease with the increasing distance from the rotating part, indicating the viscosity of the material. In the case of falling body viscometers the analysed material is in a tube and a body (ball, rod or cylinder) is fall down in it due to the gravity. The speed of falling will be proportional to the viscosity again. It can be seen that in the case of viscometers and rheometers the applied force indicates a shear stress in the material analysed. As the stress is equal to the deformation multiplied by the viscosity, the constant stress (force indicated by the mass of sample in the capillary or the rotational movement of rotational body) and the measured deformation (shear rate, falling or flowing time) will be used for the determination of viscosity. The practical use of viscosity is that it refers to chemical and technological parameters while it is easy and non-expensive to use. For example, relative viscosity between a solution and the solvent can be used for the determination of the concentration of solutes (e.g. sugar concentration), viscosity may refer to enzymatic activity (as in the case of falling number) or the flowing properties of materials within the processing or use. In the case of texture analysis pressure-tensile and cuttingshear tests are the common ones. During the pressure-tensile test a test probe with constant speed stresses the sample and the stress partially deforms the sample, partially generates a counterforce of the material. The aim of measurement can be the determination of force is required to a specific deformation, determination of the deformation caused by a specific force, the effect of deformation speed on the counterforce of the sample and the evaluation of the behavior of material in the stressing and relaxation period. An example for pressure tests is the penetrometer hardness test of apple. The cutting-shear tests try to imitate the chewing; a shear force act between the pieces of test probe on the sample placed between them (e.g. Warner-Bratzler shear force test). The results of texture analysis can be ulinked to sensory properties; cohesiveness, chewiness, springiness, hardness, adhesiveness and other sensory properties can be characterized by these tests. The last discussed are of physical properties is the electrical conductivity of materials. The materials containing electrolytes or molecules with positive and negative charges are capable to conduct electricity. As water is an electronic conductor it can be stated that all the agricultural samples conductors with a certain degree. The conductivity is influenced by the number of charges in the material (their number and their single or double charged character), and the mobility of these charges. The non-charge carrier parts of the material are impeding their circuit. This is the electrical resistance and the reciprocal of the resistance is the electrical conductivity. The Ohm’s law says that the voltage and current is a linear relationship where the proportionality factor is the conductivity (or the reciprocal of the electrical resistance). The conductivity is a stable parameter of materials only small temperature dependence have to be considered. Based on the conductivity of water, a common use of 21 Created by XMLmind XSL-FO Converter. 3. Sensory and physical quality evaluations. Role and analysis of water content electrical conductivity is the estimation of moisture content of grains and honey, monitoring the microbial growth, evaluation of fish quality and stability of fat against oxidation. 3. Role and analysis of water content Water is an essential part of living organisms, but it surrounds us everywhere as part of the inorganic materials, crystals and the air. It is medium for physiological and other processes, plays role in the formation of chemical structure, active components of chemical reactions and basic solvent. The different kinds of water can be grouped based on the type of binding. The water types with the strongest binding force are the water in the chemical structure and crystal water. These are fixed forms, so there are not bioavailable ones. The next groups are bounded by physicochemical forces – the absorbed water and water present as solvent. These forms are fixed to a specific extend, but a part of them are bioavailable. Similarly, partially fixed water type is the one bound in small capillaries by physical way. The water content bound in larger capillaries is almost free water bound with weak mechanical forces and the most easily utilizable water content is the adhering drop of water, bounded by adhesion. The availability is meant from the aspects of biological, chemical and physical kinds of processes, regardless it is useful, necessary or harmful one. The water content is the quotient of the mass of water in the sample and the mass of the sample, specified in %m/m. Based on the volume, mass, composition and structure of the material the ratios of different water types are different, thus, the binding of water is different in the case of different materials even if the water content is the same. The water activity gives information on the availability of water in the materials. It can be calculated when the vapor pressure measured in or directly above the sample is divided by the vapor pressure measured above pure water with the same conditions (temperature and pressure). Based on this term, water activity is the same as relative humidity, but the first one is characteristic to a material, the second one is to the medium around the material. To characterize the connection between water content and the water availability sorption isotherms are drawn (Figure 7). These sorption isotherms are important for storage, processing and handling. For example they show that what are the critical moisture content and relative humidity values what result rehydration, therefore favourable conditions for deterioration. Sorption isotherms help to choose the proper drying conditions. They also provide information about the possible spoilage; the different types of deteriorations have different water activity demands; the enzymatic spoilage is the most active at aw=0.9, rapidly increases to aw=0.75, and became negligible below 0.4 water activity value. The different microbes also have different demand on water activity, but the critical value is 0.6. In contrast, non-enzymatic oxidation shows high measure at low a w values and it decreases to a minimum level above 0.45 aw. 3.1. ábra - Figure 7.: Sorption isotherms 22 Created by XMLmind XSL-FO Converter. 3. Sensory and physical quality evaluations. Role and analysis of water content It can be seen that the four kinds of materials strongly difference in their water binding properties. The material marked with 1 contains significant amount of easily accessible water and 20% water content means about 0.9 a w value, thus this material is exposed to microbes, but oxidation spoilage is not typical to it. The material marked with 3 with the same water content has only 0,32 aw value; therefore the typical deteriorations is oxidation, while microbial activity is not expected. The relative humidity changes between 40 and 90% in Hungary (65-70% can be calculates on average), and the water content is equilibrium with it is about 13-14% on the 15 to 20°C temperature region. This means that the stored grains have to be dried above 14% to minimize physiological losses during storage (respiration and its consequences); higher water content will increase the risk of spoilage, but drying this lot to lower water content is superfluous, as the grains will absorb humidity from the surrounding air. The equilibrium states between water content and water activity are different when the water content is decrease or increase. In general, the same water content results lower water activity during desorption (drying) of water than during absorption (rehydration). This phenomenon is the hysteresis. Its reason is the different binding types of water and the changes on water binding places during the sorption. When a sample is being dried its organic colloids, tissue structure and other parameters change and the water binding capacities of living tissues decrease and after rehydration the ratio of weakly bounded water kinds will be higher. In the drying technology the presence of hysteresis is unfavourable and the soluble and rehydratable products require drying technology minimizes this phenomenon. Two kinds of requirements can be found regarding water content; water content and moisture content. Although these definitions seem to mean the same, there are theoretically differences can be found. Water content means the amount or ratio of H2O molecules in the material while moisture content means all the liquid parts of the sample. It is not so important when a cereal grain is analysed as its moisture content is almost water exclusively, but in the case of a fruit other liquid components are also present. Analytically, the most frequently used methods for the determination of water content is the evaporation methods, but these methods remove other volatile components from the sample what are other than water. On the other hand, the chemically bounded water content remains in the sample by most determination methods. The two main parts of a sample are the moisture content and the dry matter content in a chemical sense. When a value for moisture content or other chemical component is communicated it is important to know that this result is presented for the whole of fresh material (w0) or for the dry matter (wd) base. The calculations for these values are , where m0 is the mass of fresh (wet) sample, mw is the mass of water and md is the mass of dried sample. Based on practical considerations it is better to use the publication of result on dry matter base (when the standard or method does not require), because the results on dry matter base can be compared with each other and the moisture content may change by time – the results of measurements from different times will not present the changes of lot clearly. In the practice the report on wet base is most common for water content. The most important methods for the determination of water content are: • evaporation methods • atmospheric or vacuum heat drying Atmospheric heat drying is the most common and internationally accepted method. 2g (1 to 5 g) of sample is grinded and homogenized then dried on 103±2 °C until its weight became constant (4 hours, in general), or the temperature is 130±2 °C for specified materials. Materials which has moisture contents higher than 14 to 20%, drying with two passes is required to apply; a preliminary drying is necessary to perform on 55 to 60°C in the first pass, the mass of dried sample should measure after cooling to room temperature, then second pass can be made on 103±2 °C. The dried samples have to cool down in exsiccator before the mass measurement. It is good to remember that not only water content is determined by evaporation made by heat transfer; volatile components and other liquid what has lower boiling temperature than the applied one are also measured to the weight loss. The high temperature may causes chemical changes in the chemical 23 Created by XMLmind XSL-FO Converter. 3. Sensory and physical quality evaluations. Role and analysis of water content components and these reactions may causes other weight differences. In the case of vacuum drying the decrease of pressure causes decrease in the boiling temperature of water and makes this method not so harmful to these sensitive components. The high initial moisture content significantly increases the vapor pressure in drying oven, thus drying in two passes may necessary even if the selected method is a direct drying method. It is worth to determine the moisture contents of materials with low dry matter contents by mixing with quartz sand. • vacuum drying without heating Decrease of pressure decreases the boiling temperature of liquids, thus when it decrease under a specific value heating is not required for water desorption. Decreasing the pressure under 23.4 mbar on 20°C the water also starts evaporating. This method is friendly to the heat-sensitive compounds but also may cause losses in the volatile components. • freeze drying The pressure and temperature is changed under the triple point of water, thus the water will be present in solid and gaseous conditions, then the heating evaporates the solid water form directly to vapor by sublimation. During the process the sample is cooled and its water content freezes first, then the pressure is lowered to start the sublimation. The advantage of freeze drying is friendly but it is time-consuming and expensive. It is very rarely used for determination of water content, but more often in sample preparation. • microwave oven The energy transmitted by the microwave radiation excites the water molecules and it causes heating and evaporation while the other components are only slightly affected by this excitation. Rapid, cheap and reliable method when the sample is sufficiently uniform to provide equable distribution of energy, thereby drying. • distillation methods The principle of distillation methods are the same than those of the evaporation methods, only not the dry mass is determined, but the evaporated water is gathered, condensed and measured. Its advantage is that it has better performance for samples with low water contents and fractionated distillation can separate the water from the other volatile and evaporated components. • chemical methods • Karl Fischer titration The formula of Karl-Fisher titration is: 2 H2O + SO2 + I2→H2SO4 + 2HI. The water molecules are directly reacts with sulfur oxide and iodine, thereby only the water content of the sample can be measured. The advantage of this method is no heating occurs and electrical end-point detection improves the accuracy of measurement. • gas production methods Several gas producing chemical methods are available to the water content measurement. For example, when food sample is mixed with powdery calcium carbide, acetylene gas releases with calcium hydroxide formation by the formula: CaC2 + 2H2O → C2H2(gas) + Ca(OH)2. The determination can be made by the decrease of mass, measurement of the amount of produced gas or increase in pressure caused by the acetylene. • other physical methods • electric conductivity based methods • density based methods • water activity based methods • refractometry 24 Created by XMLmind XSL-FO Converter. 3. Sensory and physical quality evaluations. Role and analysis of water content • near infrared spectroscopy The principles of these methods were presented above. The electric conductivity based methods and NIR spectroscopy methods are current in practice; several rapid analyzers use these principles for informative analysis, but the acceptance of NIR increases nowadays; e.g. the International Association for Cereal Chemistry (ICC) has recommendation for the determination of water content by near infrared reflectance. 25 Created by XMLmind XSL-FO Converter. 4. fejezet - 4. Methods for the determination of macro chemical components of agricultural products The 95% of the body and parts of living orgasms is built from carbon, hydrogen, oxygen and nitrogen and the other 5% contains other elements, including essential and non-essential ones. The structure of chemical food analysis follows the classic classification of chemical components based on the nutritional groups of compounds (Figure 8). The component with the highest concentration in living organisms is the water; it is about 70 to 90% of total mass. The highest values can be read for the juvenile status and parts; the water contents of tissues are decreasing by age. The dry matter content can be classified into inorganic and organic groups; in the case of an agricultural product the significant part of ash content originated from inorganic contaminations. The classic analysis for determination of nutritional value of feed and food is the determination of dry matter content by evaporation method (the water content is calculated from it); then the nitrogen content by digestion or combustion methods and the protein content is calculated from this value; the fat content by extraction method and fiber content by digestion method. The remaining part is the nitrogen free extractable content from analytical considerations, containing carbohydrates, organic acids, pigments, antioxidants and other compounds. The demands of nowadays on food analysis show increasing interest on these components and the full valuation of the nutritional quality requires the knowledge on the role, amounts and principles of methods of the analysis of them. 4.1. ábra - Figure 8.: Composition of a food or feed sample 1. Nitrogen-containing compounds The nitrogen content can be present in organic and inorganic forms. The ratio of organic and inorganic forms is depends on the product, e.g. more than 95% of nitrogen is in organic form in cereal grains, but it may decrease above 50% for specific vegetables. The role of organic forms are much higher in nutrition and the presence of inorganic forms in large amounts may be harmful (e.g. nitrate content). The amino acids, as the smallest organic nitrogen compounds in agricultural products form peptides and proteins. The properties (function, nutritional value) of proteins are determined by the amino acid composition and the structure of polypeptide. Based on their forms, globular proteins (such as albumins and protamines) and fibrous proteins (e.g. myosin, collagen) can be specified. Based on their biologic function, structural, storage, transport, defensive, contractile and regulatory proteins can be listed. Simple proteins contain only amino acids while conjugated ones also contain other subunits; e.g. chromoproteins contain Fe-ion (e.g. hemoglobin), metalloproteins contain other metal ions (different enzymes) and lipoproteins contain lipid group (such has cholesterol). The derived proteins are not present naturally; they are obtained by some artificial effect (e.g. chemical, heat or radiation treatment). 26 Created by XMLmind XSL-FO Converter. 4. Methods for the determination of macro chemical components of agricultural products Analytically the simple proteins can be classified by solubility. The albumins are the most soluble fraction; they are soluble in distilled water, saline, acid and alkaline solutions, present in almost all plant and animal originated products (blood, crop grains, egg). The globulins are not soluble in water but saline, acidic and alcaline solutions they are soluble (serum and tissue, globulin). The prolamines are insoluble in water and saline and 60 to 90% alcohol-water solution, but insoluble in concentrated water or alcohol. They are typical for cereal grains. The glutelins are the most resistant to solvents; only acid and diluted alkaline solutions can solute them. They are found in plants, especially in cereal grains as storage proteins. The scleroproteins are resistant to these solvents; they are present in small amount as structural proteins and can be destructed by enzymatic way. The determination of protein content can be direct with the direct measurement of proteins in the sample matrix or indirect, when the nitrogen content is determined and it is used in formulas for the calculation. The direct methods are titration and spectrophotometric ones. The base of formaltitration method is that the amino groups in neutralized protein containing solution react with formaldehyde while proton releases. This reaction can be titrated by sodium hydroxide. The practically useable spectrophotometric methods are the UV and visible light spectrometry, from which the latter one is more common. The biuret reaction is based on that in alkaline medium the copper ion ulinks to four nitrogen atoms forming a violet complex can be measured at the 540 to 560 nm wavelength region. Its amplification is the most widely used Lowry method. The indirect methods are based on the nitrogen content of samples. Although the proteins contain 16% nitrogen content on average, the different kinds of proteins have different nitrogen contents, for example, the nitrogen content of milk and milk products is 15.67% (therefore the nitrogen content should be multiplied by 6.38), this value for corn, bean, pea and meat is 16.00 (multiplier: 6.25), for wheat grain and flour 17.15 and 17.54 (multiplier: 5.83 and 5.70), respectively, and 18.31 (multiplier: 5.46) for nut, therefore the knowledge of correct nitrogen ratio is essential for the adequate result. On the other hand, the accuracy of result depends on the method applied for the determination of nitrogen content. The Dumas method measure the total nitrogen content of sample regardless to its form. The Kjeldahl method determines the nitrogen contents of proteins, peptides, amino acids, amides and ammonia, only the oxidized inorganic forms are excluded. The amino acid analysis (AA-N) determines the amount of free and bounded amino acids and the Barnstein method measures the amount of peptides and polypeptides only (Figure 9.). As these calculated values are contains different nitrogen forms to proteins, the analytical name for this parameter is the crude protein content. 4.2. ábra - Figure 9.: Comparison of methods for the determination of nitrogen content The Dumas or combustion method determines the amount of elemental nitrogen. For this, the nitrogen containing compounds of sample are combusted at high temperature (850 to 1050°C) in the presence of oxygen and platinum catalyst and the nitrogen oxides form. The next step is the reduction of these oxides to gaseous elementary nitrogen over copper or tungsten. This gaseous product has to be clean from associated gases by absorbents and measure by volume measurement or thermal conductivity cells. The advantage of Dumas method is the simplicity, much faster than Kjeldahl method and uses only a small number and amount of chemicals, but 27 Created by XMLmind XSL-FO Converter. 4. Methods for the determination of macro chemical components of agricultural products it requires high investments and small amount of sample, therefore inadequacies occur for small nitrogen contents. The Kjeldahl method is the most common method for the measurement of crude protein content. First, the organic matter is digested by heating and sulfuric acid for one-one and a half hour, what result ammonium hydrogen sulfate. Next step is the release of nitrogen; adding an excess of base (NaOH) convert it into ammonia. The solution is boiled and the released gaseous ammonia is condensed in a receiving acid (sulfuric acid or boric acid), in which the nitrogen content is determined by titration (sodium hydroxide or sulfuric acid). This method is internationally accepted, standardized and approved, reliable, but relatively time consuming and dangerous reagents are required. The method for the determination of real protein content is the Barnstein method. First, the non-protein nitrogen content (ammonia, amids, etc) is separated by the precipitation of real protein content using copper sulfate or sodium hydroxide. This precipitation has to be cleaned from the dissolved non protein N forms by filtration and leached by water repeatedly. As a result, the cleaned precipitation contains only the nitrogen content of the proteins and peptides and the Kjeldahl or Dumas method can be applied for determine its nitrogen content. The advantage of this method is that the application of this method will result the most accurate finding on protein content, but the disadvantages are the similar to those of Kjeldahl method. It is also important to remember that the accuracies of these relative methods strongly depend on the properly chosen multiplying factor for the nitrogen-protein recalculation. Although the crude protein content is the commonly used nutritional parameter for feeds and foods its digestible part can be utilized by human and animal consumers. The digestible crude protein content can be determined by in vitro hydrolysis using pepsin, pepsin-trypsin, pancreatin or combined enzyme treatment. After specified pretreatments the samples treated with enzyme or enzymes incubated in specified temperature for required time. The quantification of solubilized proteins can be performed by Kjeldahl method or amino acid analysis. There are several other methods for the determination of protein content. Beside ultraviolet and visible light spectroscopy the near infrared and the nuclear magnetic resonance spectroscopy are widely used, the first in practice, the latter one in research primarily. Other methods, such as refractometric, polarimetric, turbidimetric and fluorometric methods are also used for special purposes, but their practical importance is minor. The evaluation of protein composition of a sample is also important to judge the nutritional value. The determination is based on the separation of the larger or smaller subunits of proteins or the analysis of amino acid composition. Relative simple method is the isoelectric precipitation based on the fact that different proteins have different isoelectric points. The adjustment of pH to specific values will determine what protein fractions precipitate and what remain in solution. The other methods require analytical instruments with high investment and operational costs and professional analysts. The adsorption methods (ion exchange chromatography, affinity chromatography) arecolumn and high performance liquid chromatographic methods, when the prepared sample is passed through a solid phase with selective absorbers and the adsorption-desorption affinity of proteins will determine their bindings. The electrophoresis uses the differences of the size and movement of proteins in an electrical field in with stationary pH (e.g. PAGE) or a pH gradient (e.g. isoelectric focusing). Size differences also can be basis of separation; dialysis, ultrafiltration and size exclusion chromatography are also suitable for the separation the proteins with different sizes. The amino acid analysis means the determination of the single amino acids in the sample. The amino acids of the proteins have to be separated by hydrolysis then they can be evaluated by ion exchange chromatography or high performance liquid chromatography. Certain amino acids can be analysed by photometric method (e.g. methionine, cystine, tryptophan). 2. Lipid content The group of lipids is a heterogeneous group of food and feed components. Their common property is that they are soluble only in organic solvents and insoluble in water. The main groups of lipids are the simple, complex and derived ones. Simple lipids are esters of fatty acids and alcohols. The fats and oils the commonly used lipids are triglycerides what are esters of fatty acids and glycerol and the waxes are esters of long chain monohydric alcohols. Complex lipids contains other components than simple lipids; phospholipids contain a phosphorus group, glycolipids a carbohydrate group, lipoproteins a protein group and sulpholipids a sulfur group. These molecules often play role in biologic processes (membrane lipids in brain tissue, myelin, occur in blood, etc.). Derived lipids are derivatives of simple and complex lipids. 28 Created by XMLmind XSL-FO Converter. 4. Methods for the determination of macro chemical components of agricultural products The fatty acids of a lipid are very important issue of qualification. The fatty acids can be saturated when there are not any double bounds and unsaturated – monounsaturated (MUFA) if contains one double bond or polyunsaturated, if it contains more than one. The land-animal (bovine, porcine) originated triglycerides contains saturated fatty acids, the main sources of MUFA are the plant originated lipids (sunflower seed, olive, canola) and the fish, nuts and seeds contain higher amount of PUFA. Unsaturated fatty acids can be cis or trans based on the arrangement of hydrogen atoms by the double bond. The trans fatty acids are rarely occurs in the nature, only the milk of ruminants. Another classification of fatty acids is by their physiological importance – some of the fatty acids (PUFAs, such as linoleic acid and arachidonic acid) are essential for animal and human organisms. The different physical and chemical properties of lipids are very important in quality and quality control. The density of fats is lower than of water. They can be solid of liquid room temperature; the solid ones are called fats and the liquid ones called oils. Lipids do not have specific melting points due to the polymorphism caused by the different properties of fatty acids. The specific gravity of fats refers to the degree of unsaturation. The iodine number (Hübl value) is a chemical quality parameter of lipids. It shows that how many grams of iodine can be taken up by 100 g of lipid. The iodine ulinks to the double bonds, therefore this value informs about the degree of unsaturation. The fatty acids dissociate from the alcohol during storage and the number of free fatty acids increases in the lipids. The acid value shows the amount of dissociated fatty acids, what can be neutralized by basis, so the filtered and dehydrated sample is dissolved in ether or benzene, then titrated by potassium hydroxide in the presence of phenolphthalein indicator. The amount of total fatty acids is characterized by the saponification value, which shows that how many grams of potassium hydrate is required to the neutralization of the whole fatty acid content of the lipid. The second chemical parameter characterizes the spoilage of lipids by the acid value is the peroxide value, which the measure of rancidity. The peroxide value characterizes the ability of lipid to liberate iodide from potassium iodine what can be measured by titration by sodium thiosulfate. The basic methods for the determination of lipid content are the extraction methods. Its first group is the solvent extraction, the most common way, what uses organic solvents for separation lipids from the water soluble compounds. First step is the sample preparation (drying, grinding and the destruction of complex lipids by acidic hydrolysis), followed by the extraction. The adequacy strongly depends on the polarity of lipids and the matching of extractant to it. Lipids with polar character require polar extractant while nonpolar lipids are more soluble in nonpolar extractant – in some cases the use of more extractants is the most appropriate way. Other requirements on extractant are the low price, safety and the most important one is that it has to be separable from the lipids completely. The separation is made by volatilization and the mass of remanent matter (extracted lipids) can be measured. Another method for the solvent extraction is the supercritical fluid extraction. This case the extractant is a heated and pressurized gas (commonly CO2), which spreads in the sample as gas but behaves as a liquid. The use of environmental friendly extractant makes this investment intensive method favourable. The nonsolvent liquid extraction methods use other chemicals for the separation of lipids from other components (proteins, carbohydrates, etc.) The most common is the Gerber method to milk. First, the lipids are released by a mixture of sulfuric acid and isoamyl alcohol what digests protein and releases the membrane lipids. After the digestion the warm (55-60°C) sample is centrifuged what makes fat to rise to the top of sample and the volume of this separated lipid can be measured. By the extraction methods other methods are also available (based on density or conductivity, or spectroscopic, NMR, X-ray absorption, light scattering methods), but the most widely accepted and approved method is the solvent extraction. The composition of lipids can be analyzed by gas chromatography what makes the separation of liquid compositions possible and can be used for determination of volatile compounds. The moving phase is gaseous or steam and the stationary phase if liquid or solid what absorbs the components of sample selectively. The extracted lipid has to be diluted in organic solvent (e.g. n-heptane) and dehydrated. An added reagent releases the fatty acids and fatty acid methyl esters are synthetized with heating and shaking. The fatty acid methyl esters are separated by the chromatograph and determined using the proper detector type. Thin layer chromatography can also be used for lipid composition analysis, when the fatty acids moves due to capillary forces and absorbed selectively based on their properties on the absorbing material. The determination is based on the distance moved by the fatty acids and its comparison to the distances moved by standards. 3. Carbohydrate content 29 Created by XMLmind XSL-FO Converter. 4. Methods for the determination of macro chemical components of agricultural products The carbohydrates can be classified to simple carbohydrates, oligosaccharides and polysaccharides in the practical approach. The simple carbohydrates are the monosaccharides and disaccharides, practically sugars. Some of them are present in high amounts in the dry matter contents of specific agricultural products (fruits, honey, sugar beet) and have significant role in the sensory properties. The oligosaccharides occur widely in plants and plant products, but their concentrations are low and have relatively low significance in quality control. The polysaccharides have two main subgroups; storage polysaccharides (starch, glycogen) and nonstarch polysaccharides what generally play role in the development of structure and acts as fibers in nutrition (cellulose, hemicellulose, lignin, pectin). The simplest physical principle of the determination of sugar content is the fact that sugar solutions rotate the polarized light in specific degree depends on temperature. This refractometric method is an inexpensive and rapid method. The chemical methods based on the chemical reactions of functional groups. The sugars can be classified to reducing and non-reducing ones; the commonly used methods based on the determination of reducing sugars due to the reactions of aldehyde group. The aldehyde group of D-glucose reduces the divalent copper ions to monovalent ones while the aldehyde group forms to carboxy group and the sodium salt of Dgluconic acid formed from D-glucose in sodium hydroxide containing medium. This is the Fehling reaction, used for the detection of reducing sugars and by the measurement of copper(I) ion concentration the amount of reducing sugars are also can be determined. The official method for the determination of sugar content is based on the Fehling reaction. First, the sugars have to leach by water, thereafter the interfering components are precipitated by Carrez I and II solutions. It is followed by ethanol addition, filtration and evaporation of ethanol and it result a stock solution. For the determination of reducing sugar content a part of it is boiled after the addition of Luff-Schoorl reagent for ten minutes, then cooled and the copper oxides precipitate. It is complemented with potassium iodide and sulfuric acid, thereafter it is titrated by sodium thiosulfate in the presence of starch indicator. For the determination of total sugar content the non-reducing sugars have to be reduced. For this, a part of the stock solution have to be acidifies by hydrochloric acid, then it has to be placed into boiling water bath after further HCl. After 20 minutes it has to be cooled down rapidly, complemented with sodium hydroxide and water and the further determination is the same to the one of reducing sugar content. The sugar composition is evaluated by liquid chromatographic methods. The commonly used for this purpose is the high performance liquid chromatography. The simplest way to evaluate the presence of starch is the iodine test; the reaction of iodine and starch result an intensive blue colouring. This simple and rapid method is widely used practice (e.g. following of maturity of some fruits) and in titrimetric. The common method is a polarimetric method: the sample is boiled in weak hydrochloric acid solution, then the proteins are precipitated by Carrez I and II solutions and the starch content of clear solution is measured by polarimeter. The starch content can be decomposed to glucose enzymatically and the determination of this glucose content is also suitable for the analysis. For some products NIR methods are also developed, but these are informative analyses only due to their reliabilities are lower. The basis of classic determination of crude fiber content is the destruction and solution of proteins, soluble carbohydrates, lipids and other non-fiber components by boiling in sulfuric acid and potassium hydroxide sequentially, then the non-soluble starches can be filtered from the dissolved components. As some of the chemically fiber compounds also dissolve, more accurate is the Van Soest method. It starts with the dilution and boiling of sample in neutral detergent solution (pH=7) what makes soluble the soluble cell components (minerals, proteins, pectine, etc.) and the remanent part is the neutral detergent fiber (NDF) containing the components of cell wall (cellulose, hemicellulose, lignin, cutin, silicon acid). This remanent is diluted in acid detergent solution what causes the dissolution of hemicellulose and the remanent is the acid detergent fiber (ADF). The dilution of this remanent leads to the dissolution of silicon acid and cellulose and the acid detergent lignin (ADL) is the remanent containing cutin and lignin. This method is widely used in feed and food analysis due to it estimated the nutritional value with good approximation and an automatized method and equipment is developed for performing. As against crude fiber content dietary fibers are the indigestible components of feeds or foods, therefore more important for nutritionists. The sample is treated enzymatically using pepsin, pancreatin and α-amilase sequentially to simulate the digestion of sample, then the supernatant and renitent fiber parts are separated by centrifugation and washing. The supernatant part is treated by amyloglucosidase and dialyzed by water and the residue is the soluble dietary fiber. The renitent part is dried and it is the insoluble dietary fiber fraction. 4. Ash content 30 Created by XMLmind XSL-FO Converter. 4. Methods for the determination of macro chemical components of agricultural products The ash content of a sample is the amount of materials cannot be incinerate at 500-600°C and the crude ash content can be determined by dry ashing or incineration on 550°C until the sample became greyish white (or at least 3 hours). Another analytical category is the hydrochloric acid insoluble ash content; this case the ash content from the incineration is boiled in hydrochloric acid, then it is filtered, dried and ashed again. Wet ashing method is also developed: the organic matter is destructed by strong acid and oxidating agent, then it ashed on 350°C. From the physical methods the ones based on electric or heat conductivity and near infrared spectroscopy are also used in practice in rapid measurements – schedule of harvest, price determination or automation in the industry. 31 Created by XMLmind XSL-FO Converter. 5. fejezet - 5. Methods for the determination of micro chemical components of agricultural products (mineral elements, vitamins, antioxidants, enzymes, organic and inorganic contaminants) 1. Mineral elements The amount of mineral elements in the agricultural products is generally low, but their concentrations are important. Their nutritional classification is based on their importance; essential, non-essential, toxic or potentially toxic elements. An element can be both essential and toxic, depending on the concentration (e.g. selenium). They can be grouped by their amount, the macronutrients have their concentration higher than 0.1% (Ca, Mg, Na, K, P, Cl and S are the essential ones) and the micronutrients or trace elements have lower ones. On the other hand, macronutrients are those ones from which at least 0.1 grams are the required daily intake for humans. Gravimetry is a relatively simple method for the quantitative determination of mineral elements. A reagent is added to the sample which selectively reacts with the element to be determined and an insoluble precipitation is formed and its dried mass can be determined. Titrimetric methods are also relatively simple to perform. It can be complexometric titration, when the cation forms a new stabile, colored complex compound with a complexing agent. The complexing agent contains a colour indicator which is displaced by the cation during titration resulting change in the colour of solution. Redox titration based on the change in the oxidation number during the reaction of sample and titration solution and this change is indicated by present specific or nonspecific indicator. During precipitation titration the specific element and titration solution forms a precipitation. The key questions of titrimetric methods are the pH of the medium, the speed of reaction and the stability of complex or precipitation. The element concentrations are also can be determined by ion selective electrodes (potentiometric method). These electrodes detect the activities of specified ions in a solution more or less selectively. The selectivity of an electrode can be characterized by its selectivity coefficient. The modern analytical measurement uses spectroscopic methods for the determination of element concentrations. Its more simple and rapid way is the colorimetry, when the concentration of a solution is determined by its comparison to a series of standard solutions. The more accurate atomic absorption spectroscopy is based on the spectrum analysis of the elements are in gaseous state and absorbs the light differently. Today the titration and atomic absorption spectroscopy are the most commonly used methods for the determination of element contents. For the spectroscopic methods the elements have to be dissolved in ionic form and before the analysis, it is required to remove the organic matrix, because it is modify the colour reaction or the parameters of spraying and the plasma. The destruction of organic compounds can be made by wet and dry methods. The wet method means digestion with strong oxidizing acids (e.g. hydrogen nitrate or sulphuric acid) and/or H 2O2 using heating. This can be performed in open system on atmospheric pressure what is simple and relatively inexpensive, but violate components may leave during digestion and endanger the environment and analysts; this can be a high temperature and high pressure closed system, what is more suitable for the determination of elements present in lower concentrations, the closed system does not result losses, but it is relatively time consuming comparing to the open system; or this can be closed digestion with microwave heating, what also does not result losses, but more rapid and energy saver comparing to the previous closed system. The second way is the dry sample preparation the sample is ashed (500-600°C), then it is dissolved in acid. This method is inexpensive, more 32 Created by XMLmind XSL-FO Converter. 5. Methods for the determination of micro chemical components of agricultural products (mineral elements, vitamins, antioxidants, enzymes, organic and inorganic contaminants) simple and rapid for large number of samples, but the incidence of losses is higher in comparison to the wet sample preparation. The prepared sample is induced by electromagnetic radiation. Due to the induction, the electrons of the atoms have an instable higher energy level and rapidly recombine to a baseline and lead the surplus energy by emission. The detection of this emitted light results a thermodiagram and its analysis and comparison to the baselines of different elements (the wavelength what is the most characteristics of the elements) are the general principle for the quantitative determination. The spectroscopy can be performed emission or transmission method; the first one evaluates the emitted, the latter one the absorbed light. The emission based methods are the flame and plasma emission spectroscopy. In the case of flame photometry the induction is made by flame; the sample is sprayed into flame, then the emitted light is divided spectrally and amplified, then the luminous intensity on specific wavelength is measured. In the case of plasma emission, a plasma with 6000-8000°K is generated by high frequency current in a reel. The sample is atomized, then carrier gas carries it into the plasma when it ionized, resulting huge increase in the energy of electrons. Comparing flame and plasma emission spectroscopy, the flame photometry is more simple and inexpensive, but the plasma emission has much higher accuracy, much lower matrix-effect occurs and multielemental analysis can be performed by this method. Its jointing to mass spectrometer very low detection limits can be achieved. The absorption spectroscopy can be done by atomic absorption photometry, when the absorption of atoms is evaluated and by UV and VIS absorption photometry, when the absorption of chemical bond is measured, therefore it is rather used for the determination of organic components. The wavelength of absorbed light refers to the kind of molecule, atom or bond and the amount of absorbed light refers to the concentration. Atomic absorption spectroscopy can be used for the determination of elements in very low amounts in sample. The induction can be induced by flaming and electrothermic reaction. 2. Vitamins and provitamins Vitamins are those organic components of foods and feedstuffs what are essential for human and animal organisms. They are mostly synthetized in plants, but vitamin C is synthetized by animals other than monkey and guinea pig from glucose. Provitamines are those compounds what can be transformed into vitamins in the organisms and antivitamined are those compounds what hinder the organisms in utilization of vitamin by their bounding to the enzyme displacing the vitamin. Their adequate consumption is important, because both overdosage and lack of them have symptoms and risks. Their basic classification based on their solubility; water and lipid soluble vitamins are the two main groups. Their chemical structure is so diverse that there are no general determination methods but all kinds require different sample preparation and determination. The similarities in the analysis are that the low concentration to determine and their sensitivity to external factors, e.g. heat, light, oxygen and others what makes the analysis complicated. The general way of the quantitative determination of vitamins starts with extraction by water for water soluble ones or organic solvent for lipid soluble ones. The extract has to be purified from disturbing agents by chromatographic separation techniques generally, then the determination is performed by titrimetric, spectrophotometric, fluorimetric or chromatographic methods. An example for the determination of lipid soluble vitamins is the method for the quantification of vitamin D3 content. This case the extractant is methanol; the sample is weighted to an Erlenmeyer flask, after the addition of methanol the flask has to be closed and shaken in ultrasonic shaker until the extraction is done. The liquid and solid phases have to be separated by vacuum filtering or centrifugation, then the cleaned sample has to be measured by HPLC using specific eluent, flowing speed and retention time. The vitamin C content from the water soluble vitamins can be determined by titrimetric, photometric and separation technic methods. The titrimetric method can be iodometric one; this case the ascorbic acid content is extracted by metaphosphoric acid or oxalic acid, then the liquid phase is filtered, completed with starch indicator and KIO3. and titrated with KI to permanent blue colour. The potassium iodine oxidizes the ascorbic acid to dehydroascorbic acid and if it ends, it oxidizes the potassium iodate, and the releasing iodine makes blue complex with starch molecules. Another titrimetric method is the 2,6-dichlorophenol-indophenol titration, when ascorbic acid is extracted from the sample by oxalic acid or a mixture of metaphosphoric acid and acetic acid, and the extract is titrated by 2,6-dichlorophenol-indophenol until reaching salmon pink colour. The 33 Created by XMLmind XSL-FO Converter. 5. Methods for the determination of micro chemical components of agricultural products (mineral elements, vitamins, antioxidants, enzymes, organic and inorganic contaminants) spectrophotometric method starts with the same extraction as in the 2,6-dichlorophenol-indophenol titration, then the liquid phase is filtered, diluted and completed with sodium acetate-acetic acid buffer solution and 2,6dichlorphenol indophenol solution, mixed and centrifuged, then the absorbance of the upper xilene phase is measured and compared to standards. The ascorbic acid concentration also can be determined by HPLC; in this case methanol-phosphate puffer is the extractant and the extract directly injected into HPLC using electrochemical detection. 3. Antoxidants The measurement of antioxidant concentration is also a complex issue. The total antioxidant content is the sum of the concentration of different antioxidants, but the different kinds require different methods and their comparison is rarely possible. In practice, the concentrations of different antioxidants (e.g. phenolic compounds, tannins) can be measured by photometry, titrimetry, HPLC, etc. From physiologic aspects, more important parameter is the total antioxidant activity, summarizes the cumulative antioxidant effects of compounds of this heterogeneous group. The total antioxidant activity can be determined by indirect and direct methods. The indirect method is the measurement of the free radical scavening activity of the sample, when the sample is activated with stable free radicals, then the decreased light intensity of radical is determined by photometric ways. The direct method is on the based the evaluation of the stability of lipid substrates containing antioxidants. The direct methods are more sensitive and accurate than the indirect ones, but much harder to carry out. Indirect methods only estimate the antioxidant effect due to the in vivo effects of matrix, but much more easy to perform, rapid and effective. The sample preparation aimed to help releasing antioxidants for the measurement. Generally it starts with shredding or grinding of the sample but – similarly to the vitamins – antioxidants are sensitive to heat, oxygen and oxidizing agents, thus their protection is important. For this the size reduction is made under the extractant, therefore the size reduction and the extraction start simultaneously. The recently used extractant are organic ones; methanol, mixture of methanol and water, acetone, dichloromethane and in some cases water. After the antioxidant content is extracted to the liquid phase, separation (centrifugation or filtration) is used for producing stock solution. To quantify the antioxidant activity, total radical scavanger capacity (TRSC) method and electron transfer based methods are the common ones. The TRSC method is based on the phenomenon that the luminol is oxidized by hydrogen peroxide in alcalic medium while it emits light. This luminous intensity can be measured by photometric methods. Addition of prepared sample containing antioxidants inhibits light emission and the rate of inhibition refers to the reduction-oxidation properties. To quantify it antioxidant standards are used for reference. The electron transfer based methods determine the reducing power of the sample. This way, total antioxidant capacity is determined in the proportion of a known antioxidant (e.g. ascorbic acid, catechin). Added oxidizing agent or free radical respond to the antioxidants of the matrix and this reaction can be characterized by spectrophotometric measurement. The advantages of these analyses are that they are rapid, simple and relatively inexpensive methods. For example the ferric reducing antioxidant power (FRAP), trolox equivalent antioxidant capacity (TEAC), oxygen radical absorption capacity (ORAC) and 1,1-diphenyl-2-picrylhydrazyl (DPPH) assays are based on this principle. The comparability of the results of the different methods is not full, therefore the valuation of antioxidant activity values have to include the applied method and the extractant. 4. Enzymes Enzymes are macromolecules, proteins, regulate and catalyse the chemical and biochemical processes is living organisms. Their activity is required for physiologic processes, but after harvest or slaughter or during storage it continuously decreases what is generally favourable as these biochemical processes are often decrease quality and causes losses. For example the activity of polygalacturonase and pectinesterase results tissue changes (softening) of fruits decreasing shelf-life, the activity of chlorophyllase and polyphenoloxidase result change in the colour in vegetables, the lipoxygenase and lipase result rancidity and off-odour and off-flavor production for foods rich in lipids and the amylases result losses in carbohydrate content. On the other hand, their activity is required in several cases, e.g. the work of amylases is necessary for bread making and the maturity processes of 34 Created by XMLmind XSL-FO Converter. 5. Methods for the determination of micro chemical components of agricultural products (mineral elements, vitamins, antioxidants, enzymes, organic and inorganic contaminants) climacteric fruits. Enzyme activity analysis is also suitable to the simple check of technological processes; e.g. the efficiency of heat treatment on soybean by the analysis of urease activity or the efficiency of blanching of pea by the evaluation of guaiacol-peroxidase activity. The measurements of enzyme activity can aim to determine the active presence of an enzyme (qualitative) or determine the current activity (qualitative). Enzyme activity can be specified numerically when the purpose of the analysis is its accurate quantification. Its SI unit is kat what means catalytic activity; the transformation of substrate per second (i.e. mol/s) but U (unit) is also used in analytical practice. The used chemical method can be titrimetric, calorimetric, photometric, chemiluminescent, light scattering, radiometric or chromatographic one. For the agricultural products relative determination are also common, where the speed or any other measurable result of a process is determined and in some cases compared to a standardized value. The relative determinations are more rapid and chemical saving methods and based on a simple chemical, physical or biological principle, this is why they are preferred in practice. A very simple practically used relative physical method for the amylase activity measurement of cereals (especially wheat and rye) is the Hagberg’s falling number. Flour or groats with about double amount of distilled water placed into a viscosimetric tube, closing with rubber stopper and making a homogeneous suspension with intensive shaking. The tube with the mixing stick is placed into a boiling water bath and the equipment starts to mix the suspension and measure the elapsed time. After 60 seconds of mixing, the mixing stick is dropped at the top position and it start to sink down to the bottom of tube. The speed of singing down depends on the resistance of gelatinizing starch and the activity of amylase. The time from the beginning of mixing to the stick reaches the bottom of tube is the falling number, measured in seconds. The amylases activated by water addition and heat start to break down the starch and the favourable conditions help to reach the maximum enzyme activity, thus, the enzyme activity of sample will determine the speed of liquification. Higher amylase activity results faster liquification and sooner end of test, hereby the low falling number means higher amylase activity. 5. Organic and inorganic contaminants The contaminants of agricultural products can be classified into organic and inorganic groups (Table 1.). The inorganic contaminants are physical impurities (rock, metal, soil) and metal and heavy metal ions and compounds and nitrate. The analyses of the physical impurities were discussed in sensory analysis, the methods for determination of contaminant elements are the same to those of mineral elements. The nitrate content is easily and selectively can be determined by spectrophotometric methods in general. 5.1. táblázat - Table 1.: Organic and inorganic contaminants of food and forage Source Organic Inorganic Metals (Cd, Hg,…) Environmen Microbes and toxins t Agrochemical residues Human activity Rock, stone, soil Agrochemicals (residues of pesticide and growth regulators, not Agrochemicals (nitrate) allowed chemicals) Animal drugs, hormones GMO Processing Packaging materials Metals Processing contaminants Physical contaminants Microbes and toxins 35 Created by XMLmind XSL-FO Converter. 5. Methods for the determination of micro chemical components of agricultural products (mineral elements, vitamins, antioxidants, enzymes, organic and inorganic contaminants) New components: acrylamide, histamine, furane, etc. The mycotoxins are the most important natural organic contaminants of agricultural products. They are secondary metabolites of fungi, present in the samples in very low amounts and have serious health effects. Alfatoxin (maize, peanuts, spices), ochratoxin (fruits, fruit juices), ergot (cereals), patulin (fruits and vegetables) and toxins of Fusarium species (fumorisin, trichotecenesand zearalenone; cereals) have practically significance. For the determination qualitative and quantitative methods are also developed. The obvious test might be the mycologic and microbiological evaluations, but the presence of fungi does not mean that toxins are also present as optimal external conditions are also required for production (and the presence of toxins does not prove the presence of microbes, however). Biological evaluations (biosensors) are rapid, easy-to-use and inexpensive methods, based on biological reactions what are amplified and transformed into electronic signal. The enzymatic evaluations based on specific ulinking between enzymes and substrates. In the case of enzyme-ulinked immunosorbent assays (ELISAs) the toxins present in the sample and added toxins compete for the binding places. The labeled toxin binding results change in absorbance of sample, therefore the lower toxin content in the sample results higher binding of labeled toxin, therefore higher absorbance what can be quantified. The third approved method is chemical analysis them the toxin content (if it is present) is extracted by organic extractant (e.g. aqueous acetonitrile or aqueous methanol for the extraction of deoxynivalenol) then the extract is cleaned up (by liquid–liquid partitioning, solid-phase extraction, column chromatography, use of immunoaffinity columns and multifunctional clean-up columns) then the toxin content of purified sample is separated and detected by ELISA, chromatography (TLC, GC, HPLC, LC-MS) or UV and IR spectrometric methods. Experimental results are available about the utilization of NIR method for screening mycotoxins in cereals, but it is only in preliminary status and only can be used for qualitative screening for specified toxins. The Fluorescence Labelled Optical-Read Immuno Dipstick Assay (FLORIDA) method uses special fluorescent complexes what have high sensitivities in screening. The main aim of this subject is to expound schematically the general analytical methods. There are several books and online references are available for the students who want to get more information about the chemical analysis and the references section also contains useful sources for them. 36 Created by XMLmind XSL-FO Converter. 6. fejezet - 6. Microbiological analysis of agricultural products Microbiological analysis is the third part of quality control of agricultural products by the physical and chemical ones. The presence of microbes can be required; fermented food products are made by the contribution of favourable microorganisms (lactic acid fermentation, acetous fermentation, alcoholic fermentation) or they improve our digestion and provide effective nutrients by the utilization of prebiotics in the digestion system. On the other hand, the harmful ones cause deterioration; these microbes are present everywhere in the food chain from the soil to our table. The judgment of a microorganism depends on the purpose; a lactic acid one is useful during ensilation or leavened cucumber making but harmful during acidification of a horticultural product or pickled cucumber making. The most important for quality control regarding microbes is food safety; both presence and toxins are dangerous contaminants of feeds and foods. Internal and external factors of product influence both the kinds and number of present or potentially present microbes. Its basic chemical composition primarily determines what microbes grow on it; the lack of required substrate or the presence of retardant are equally important to set up a hypothesis on the microbe composition. Added chemicals (from the natural sugar or acetic acid to the artificial preservatives) may significantly influence the microflora – this is a basis of food preservation. The other internal parameters are the water activity of the product (the general limit value for microbes is 0.6), pH (an important value is 4.5; this is the limit pH under what the growth of Clostridium botulinum is inhibited and a slighter heat treatment is required for the preservation of canned products) and the oxidation-reduction potential. The most important external factors are the temperature and gas composition of medium; most of the present pathogens are aerobic ones, but some of the anaerobic ones are highly dangerous (e.g. the Clostridium species). The microbiologic analysis can aim the analysis of microbes or the evaluation of product. From the aspect of microbes the purpose can be the determination of the total count of microbes or a specific group (e.g. molds), the evaluation of the presence of a selected one or the analysis can aim to analyse the types of present microbes. From the side of product, the analysis can aim to determine its microbiological purity, making decision on the suitability or acceptance, or the evaluation or control of technological process (fermentation industry). In every cases, the microbes are living components of sample and the environment of sample, therefore much more carefully should be exercised in the sampling, the storage of sample and the analysis 1. Sampling for microbiological analysis General principle of sampling is that the sampling process should not contaminate the sample. In the case of sampling for microbial analysis, the sampling tools and packaging materials have to be sterile and nontransparent. Based on the purpose of analysis, the assessment of the general microbial status of product or exploration of a possible contamination require different sampling plan to make. Large lots are much more heterogeneous for microbial properties than for chemical properties; e.g. the differences of parts of a grain lot in micro climate may cause significant differences in microbial activity, composition and number. On the other hand, when a bulk sample is collected the heterogeneity of lot disappears and the sample will prove the suitability of the lot despite it is partially unsuitable. The sample container must not be overfilled, it should be filled only to its fifth-sixth volume. If other analyses are not purposed, the size of a food or feed sample must be about 200 g or ml. The samples must be stored and transferred on 4°C except the analysis or the sampling plan do not prescribe other conditions. The samples have to be transferred into the laboratory as soon as it is possible; generally the maximum allowed time between sampling and evaluation is 24 hours. The sample preparation has to be performed only just before the analysis. The solid sample must be grinded and mixed and measure 10 g for the examination, the liquid sample must be mixed well and leave to ensure the rising and leaving of blebs and measure 10 ml for the analysis. 2. Methods of microbiological analysis The analysis can be qualitative when the composition of microbes or the presence of specific microbes is aimed to determine or enumeration rests when the microbe numbers have to be determined. The qualitative tests can be microscopic when simple visual evaluation by magnification of sample is performed. The general way of analysis is the same for the test with different purposes (Figure 10.). First, the food or feed sample is mixed and 37 Created by XMLmind XSL-FO Converter. 6. Microbiological analysis of agricultural products homogenized with a specific buffer, then it engrafted in a nonselective media. This step is a nonselective preenrichment stage aimed to resuscitate the microbes present in the sample. After an incubation period, the grown colony is sampled again and engrafted in a selective media to help developing the examined microbes (selection stage). After a second incubation period the separated colonies can be separated to grown media what makes possible the selective quantification (propagation postenrichment). The colonies grown on the nonselective, selective or grown media are incubated on differential and selective agars from where cleaned items can be picked for biochemical testing firstly aimed to identification, then serotyping for characterization. 6.1. ábra - Figure 10.: Conventional microbiological analysis of foods (Feng, 2007) 3. Qualitative determination of microbes The most simple and inexpensive method is the microscopic analysis using compound light microscope. This case the grown microbes are evaluated visually using the magnification of objective and ocular lens. The object lens collect the light from the direction of sample and forward to the ocular lens production an image of the object. The magnifications of objective lens are higher (generally 5 to 15-fold higher) than those of the ocular lens. The maximum reachable magnification is about 1500-fold, that is its resolution is 0.2 micrometers. This method is rapid, but can be applied for estimation and large numbers of present microbes can be determined only (above 106 /ml). It can be used for the counting of both viable and non-viable cells. In the practice it is used mainly for the analysis of yeasts and molds, and possibly for bacteria (above 1 micrometer size). The microscopic method can be dark bright field or field one. The bright field microscopes the lighting is from below, the object (microbes) absorb a specific amount and the others are passed through to the lens with the scattered light. Therefore, the background is bright. As the microbes have a low light absorbing capacity, the difference between the light from the object and the scattered light is very small and this makes difficult to examine. The visibility of cells can be improved by staining. The microscopic analysis of stained cells commonly uses Gram staining. The analysis requires clean degreased slide, as these kinds of contaminations strongly influences the scatter of light. The microbes have to be suspended in liquid for the analysis, therefore liquid samples can be applied by simple dropping to the slide, but solid samples have to be suspended in water or specific solution. After the smear dries, the fixation is the next step when the plate with dried sample is placed above the flame of Bunsen burner. The living cells die, the proteins precipitate and became free to stain. The colourization is staining with crystal violet solution and fixing with iodine solution, then the excess of stain has to be washed down with alcohol or acetone and dried. It is followed by a post-staining with safrarin, surface cleaning (washing down the slide with water) and drying (wiping the remaining moisture with filter paper). As a result, viewing the slide with microscope (1000x magnification) the Gram negative microbes show crystal violet colour while the Gram negatives remain purple The dark field microscopes receive the rays of light from a cone light source, therefore the unscattered light will be excluded from the image, leaving dark the background of it. The cells of microbes will shine brightly in the 38 Created by XMLmind XSL-FO Converter. 6. Microbiological analysis of agricultural products image and it results much better resolution (objects with their size less than 20 nanometer) and reading conditions. Specific compounds illuminated with high energy – small wave length light emit light with lower frequency. This is the phenomenon of fluorescence what is an opportunity for location and identification of molecules (proteins, pigments, etc.) in the cell makes identification possible. The fluorescence increases the sensitivity of detection and straining of different molecules expands the analytical possibilities. This principle is used by fluorescent microscopy. The most common stains used in the microbiological analysis are acridine orange, DAPI (4',6-diamidino-2-phenylindole), ethidium bromide and rhodamine, and their usage highly increases the sensitivity of reading. The liquid sample has to be dropped on the slide, dried, fixed with methanol, then dried with air again. It is followed by staining with the molecule or compound specific stain, the excess is rinsed with water and dried again. The dried plate can be examined by fluorescent microscope. As the wavelength of emitted light is different to the illuminating light, the image shows only the emitted one (the compounds are analyzed) and the specificity can be increased by using multicomponent staining and light filters. Nowadays this is the most common screening procedure, due to its simplicity, rapidness and sensitivity. The resolution of microscopic image is limited by the properties of light, therefore it cannot be decreased below a specific value. Better resolution can be achieved using electron microscopic methods, what has thousand-fold higher resolution comparing to the value for light microscope. The imaging is made by accelerated electron beam, emitted by an electron gun, what is focused by consider lens to the specimen what scatter it again. The objective lens collect and focus the electron beam and form a real image, what can be projected to a phosphorescent plate what makes it visible for human’s eye. The lens applied in electron microscopes are not commonly understood lens, but magnetic or electromagnetic fields what capable to collect and focus the electrons in the specific degree. The interior of the electron microscope is under vacuum, due to the electrons have very small masses and despite of their high velocity, collusion with any molecules diverts it and worsen the imaging. Therefore, the sample has to be dried as the water content of the cells evaporates under vacuum rapidly resulting destruction in structure of cells. Drying has to be gentle to avoid shrinkage and collapse, and the original structure is maintained. Specific structure fixing methods can be applied. Using freeze etching, fixing and drying are not necessary; in this case the specimen is quick frozen by liquid nitrogen at -190°C resulting very small ice crystals what minimize structural changes (due to the small sizes and lack of osmotic processes of freezing) and results fixed structure (due to the formed crystals – no shrinkage and collapse). The ice sublimates under vacuum without resulting changes in cells (by the same considerations as for freeze drying).To achieve a real two dimensional image from the specimen, thins sectioning is necessary avoiding the projection of different layers onto the image. In general, slices with 100 micrometer thickness are suitable. While using microscopic methods the individual microbes are evaluated, culture techniques examine the microbe populations. These methods are indirect and time consuming evaluations, but much selective and accurate ones and can be used for both qualification and quantification. The microbes are grown under controlled conditions (water activity, nutrients, pH, air composition, etc.) and the favourable conditions for multiplication cause rapid cell number. The medium used in culture techniques contains obligatory and conditionally components. The four obligatory components are: • water • carbon and energy source (mostly sugars, peptone, meat extract) • nitrogen source (mostly ammonium, nitrite and nitrate salts (inorganic), or neat extract, natural proteins, peptones, triptones and amino acids (organic) • minerals (inorganic salts) The kinds of conditionally components depend on the culture to be grown and the aim of evaluation. The most frequent ones are the different vitamins, elements and organic and inorganic electron donors and acceptors. The media containing only mineral sources (and in some cases carbon) are called minimal media. The addition of specific components provides opportunity for microbes to grow which requires that component and the type of energy source (added carbon or light for photosynthetic microbes) are also selects from the microorganisms. Complex media contain much more organic components and provide suitable growing conditions for several 39 Created by XMLmind XSL-FO Converter. 6. Microbiological analysis of agricultural products microbes, but the present lurking microbes also can be cultivated by the additions of their specific limiting grown factors. Complex media is more frequently used for general analysis of agricultural samples. The media can be classified by other practical considerations: • composition • natural: contains only natural materials (clear soup, molasses) • semi synthetic: natural originated culture is supplemented by synthetically produced components (e.g. agar media) • synthetic: contains only artificially produced components (commercial products) • condition • liquid: used for growing a culture with high number or the inoculum presumably contains only low number of microbes, but only one type of microbe can be detected. • semi-liquid: helps to separate motile and non-motile strains • solid: only one cell or colony forming produces a pure culture usually on the surface or inside the medium. • aim • base medium: it is the most simple one and allows to grow the microbes having no specific grown factor. • elective medium: its composition limits what kinds of microbes can grow on the plate (supplemented base medium). • selective medium: its composition helps specific microbes to grown and inhibits others. Practically, selective medium helps pathogen against commensal ones. • differential or indicator medium: it helps the visual distinction of specific colonies by their colour. • resuscitation medium: it makes possible to grown such microbes which lost their reproduction ability due to any environmental inhibition factor. • special medium: its composition helps to grow only a specific type of microbes. The culturing starts with the preparation of medium. Several types are readily available but specific ones have to be prepared or supplemented. It starts with the preparation of raw materials and measuring the required components. Subsequently, the components are dissolved in the order written in the recipe with continuous mixing. When the homogeneous mixture is ready, pH measurement (by temperature compensation pH meter) and adjustment (with 1n NaOH, Na2CO3 or HCl, citric acid, malic acid or other agent) are the following steps. Then the medium is filled into the container, flask or plate to the final volume, and if the medium is not liquid, the controlling of firmness of solid and semi-liquid medium is also important and if necessary, it has to be adjusted. Then the medium is ready it has to be packed and sterilized in autoclave (115-121°C, 15 min or fractionated sterilization; 80-85°C, 1-2 hours, 3 times or with membrane filtering) and stored in dry, dark and cold (between 4 and 8°C) place. The microbes are required facultative or obligatory aerobic and anaerobic conditions for growing, therefore the incubation also can be aerobic or anaerobic. For the aerobes the culturing can be performed in normal air conditions, and in liquid media they can use the dissolved oxygen, however, due to the high water activity and relatively slow diffusion of oxygen their growing may slows down. Shaking and bubbling can maintain the aerobic condition in this case. In the case of solid or semi liquid media there are different conditions on the surface and inside; the inner layers are continuously became poorer due to the lack of oxygen. In the case of anaerobic culturing the air has to be excluded. It can be performed physically with shutting out of air by paraffin oil or paraffin stopper or using of anaerobic jars or thermostat. The use of gooseneck on the flask also helps shutting out the resupply of oxygen. Chemical methods are also available for the depletion of oxygen by specific reactions. The aerobic conditions are also can be hindered by the use of added reducing agents (e.g. mercapto or –SH groups). 40 Created by XMLmind XSL-FO Converter. 6. Microbiological analysis of agricultural products There are several isolation techniques are available for the implementation and evaluation of culturing. The most simple is the serial dilution, when the sample is diluted stepwise following a predefined dilution factor. It is usually ten in microbiologic analysis (serial decimal dilution). This case the required amount of liquid sample is placed from a flask to the plate and a second part of it is diluted to tenfold using an another flask. From this second flask a part is transferred to a plate and an another part is to the next flask (hundredfold dilution). This dilution and inoculation sequence has to be continued as it is necessary. The advantage of this method is that the analysis can be used in a wide range of cell number and the plate of previous and following dilution helps checking the result. The pour plate method is starts with the preparation and dilution of sample, what is transferred to the sterilized plate. The medium has not higher temperature than 40°C is poured on it and mixed with the sample well, then the homogenized sample and medium solidifies and became ready for incubation. The advantage of this technique is no special preparation is necessary, but the growing of aerobes is slower in the inner layers of medium. This problem is solved using spread plate method, when the prepared liquid sample is transferred to the surface of the solidified medium and spread using a sterilized glass rod. In the case of Miles and Misra technique, the plates containing solid medium is divided into equal sectors (generally 6 or 8) and a previously prepared serially diluted sample is dropped to the proper sector and let drying naturally. After the incubation period the different dilutions can be examined simultaneously, excluding the possibility of error due to the heterogeneous media. Similarly to this, the use of streaking method expands the examined concentration range. The surface of solid medium is divided into parts and the diluted sample is applied on one part of it. The first inoculation probably contains too much number of microbes, using a sterilized loop a part of transferred sample is dragged to an another part of plate and this procedure repeated as time as it is necessary. The dragging decreases the number of cells in the newly inoculated parts making possible the rapid identification. In the case of determination of Most Probable Number (MPN) a decimal dilution sample sequence has to be prepared until the probability of the presence of cells of specific microbe is zero. The dilutions are transferred to the media. After the incubation, the number of positive and negative inoculation results is determined and the most probable number can be determined using the Hoskins’ table. The method is adoptable when the distribution of microbes is homogeneous and random. The identification of microbes can be morphologic when their appearances are evaluated, metabolic when their metabolisms are the base of identification or serologic when the specific added antibodies react with the antigens. The morphologic identification can be micro-morphologic when the examination of the presences or absences of specific endospores, flagella, glycocalyx, etc., so the different parts of the cells are the basis for identification. These analyses are used in microscopic examinations. The macro-morphologic evaluations examine the cells or particles higher than cells. For microscopic analysis the cell shape and size can be a basis. For culture techniques several morphologic aspects can be considered: • the shape of colony on solid medium: shape in top view, side view, edge of colony in top view • the nature of colony: rough; smooth; medium • the shape of colony in liquid medium • multiplication in the medium: diffuse clouding; sedimentation; flocculation • multiplication on the surface of medium: skimming; pellicula formation; homocentric colony formation The metabolic identification for the microbial analysis of food and feed are based on the evaluation of carbohydrate and nitrogen metabolism or the activity of extracellular enzymes. The carbohydrate metabolism evaluation can be based on • the evaluation of used carbohydrates by the comparison of the composition of medium and the evaluation of raised acids and gases • the acid production of microbes – methyl orange test • how does the microbe use the carbohydrates – oxidative-fermentative (OF) test 41 Created by XMLmind XSL-FO Converter. 6. Microbiological analysis of agricultural products • how strong is the reducing effect of microbe? – differential media • how does the terminal oxidation enact - catalase or oxidase test Evaluation of nitrogen metabolism is based on the evaluation whether the microbes reduces the nitrate or not (nitrate reduction test), whether they dissociate urea or not (urease test) or whether they produce indol or not (indol test). The evaluation of extracellular enzymes can be the evaluation of lipase, lecitinase, coagulase or hemolysin activity. In the case of serologic identification a specific antibody is added to the medium and the antigen answer is examined. Generally the presence of a specific microbe species is determined by classic methods (culture technique or microscopy) and the variety or varieties are identified serologically. The positive result of an agglutination tests means that corpuscular antigenes engage with antibodies and visible agglutinations appear. Another kind is the precipitation test when the dissolved antibody forms an immune complex with an antigen and it separate out as precipitation when the test is positive. The third kind is the complement fixation tests when the antibody forms an immune complex with an antigen when the test is positive and this complex is engaged with a selective complement. 4. Other microbiological methods From physical methods, the impedance measurement can be used for the determination of cell number from 10100 cells (to 104to 106/ml concentration). It based on the monitoring of impedance changes by time as an effect of microbial metabolism. The calorimetric methods calculates microbial activity based on the produced heat of metabolic processes, therefore the measurement of heat flow with batch and flow calorimeters is also suitable for the analysis. The principle of flow cytometry is the same to that of turbidity; monochromatic light is directed into the liquid sample what is translucent. The cells of microbes scatter light beam and the forward and side scatter is suitable for characterization of the type and amount cells. In the case of radiometric methods culture medium with marked carbon isotopes (e.g. in glucose) is used by microbes and it can be measured in the fermentation product. The dye reductions tests use simple principles. The test starts specific dye addition to the liquid sample the present microbe changes its colour while using oxygen. For example, methylene blue dye addition to milk colours milk but due to the bacterial activity it loses its colour. The speed of decolourization refers to their amount. The ATP determination method is based on the phenomenon of bioluminescence. A special lysing agent is added to the microbe suspension what breaks down the cell walls and release the content of cells, including the ATP. Luciferrin is also added and it reacts upon the ATP in the presence of O2 with light emission. The light emission is proportional to the amount of ATP, therefore the number of cells. ELISA (enzyme-ulinked immunosorbent assay) is an immunological method when an antibody labelled with enzyme is added to the medium containing bacteria. The reaction is not influenced by the enzyme, but tracks it. The antibody bind to the antigen and the enzyme helps to present the result by colouring. Immuno- and biosensors are also can be used for determination. In this case, analytical devices are used for the detection of analytes that combines a biological component with a physicochemical transducer. The biological component reacts with the present specific compound of or produced by microbes and the occurrence of this reaction makes the transducer to produce measurable signal, proportional to the amount of analyte. The analysis of genetic information of microbes is also suitable for qualitative and quantitative analysis. Gel electrophoresis can be used for separation of DNA, RNA or protein using an electric field based on size, shape or electric charge. The order of bases in DNA determined by DNA sequencing also unequivocally characterises the species. The PCR (Polymerase Chain Reaction), the real-time PCR (qPCR) is a method when the amplification of a single DNA fragment (up to ~10 kilo base pairs) for isolation, identification and quantification is performed. Blotting (southern, northern, western, eastern) is also a method for detection the presence of microbial marks (DNA sequences, RNA sequences, protein molecules or modifications. The DNA hibridization is the evaluation of similarity of pools of different DNA. The double helix of DNA is separated to two lines with heat treatment and bacterial DNA part is added to it. The strength of reaction between the two lines refers to the relatedness. 42 Created by XMLmind XSL-FO Converter. 7. fejezet - 7. Requirements for cereals and their products (Physical, chemical and functional properties) I. Cereals are grasses with grains suitable for consumption. They are staple corps; provide more amount of energy from carbohydrates for humans and animals than other crops do. In addition, they are rich in proteins, fibers, minerals, vitamins and antioxidants, but their nutritional value is not sufficiently exploited due to the modern consumption habits; the separation of bran and germ from the generally consumed endosperm part removes the most valuable nutrients. 1. Quality of wheat The quality parameters of wheat can be classified into four groups; physical, chemical, rheological and food safety related ones. On the other hand, from the view of industrial use, technological quality parameters are the most important ones and them are came from these groups; purity, gluten content, galling number and rheological properties. Nevertheless, it is better to follow the classic classification what can help to understand the technological importance. General requirements The general requirements on wheat contain all the basic expectations what all the lots offered for sale or storage have to be fulfill. They have to be dry enough avoiding the losses and spoilage caused by the high moisture content. They have to be healthy, free from pests and sieved sifted as much as necessary for long-term storage. The parts of the lot have to be originated from the same cropyear and, as far as it is possible, have to contain grains of the same varieties or variety groups providing homogeneous parameters. 2. Physical parameters The physical properties are the organoleptic properties, purity and the hectoliter weight. The organoleptic properties are important marks of the health status and homogeneity. This includes: • colour: it has to be typical of the healthy wheat grain, rubescent-brown (red wheat varieties), but brighter marks rubescent-brown to tawny are allowed. The surface of grain can be lustre of lustreless, typical of variety. • odor: it also has to be typical of the healthy wheat grain. Moldy, fermented, close and other strange odor is not allowed. • smell: wheat grains have a typical saccharine taste, sour taste is not allowed. The evaluation of purity of a lot shows that what kind of foreign matters are present in it and in what extend. The different impurities in a cereal lot can be valuable (e.g. small amount of rye improves the baking quality of wheat flour or non-toxic grains with nutritional value are valuable impurities for feed use), valueless (e.g. grains of other cereals in the wheat lot for baking industry) or harmful (e.g. toxic weed seeds). The Regulation No 824/2000 of 19 April 2000 of European Commission distinguishes the following groups of impurities for the lots offered for intervention buy-up: 1. Broken grains: those ones in which a part of surface uncovered and the endosperm can be seen. Their total rate should not exceed 5% 2. Grain impurities contains shrivelled grains (those wheat grains which passes through a sieve with apertures of 2.0 mm), other cereals (all the cereal grains what are not the same species), grains damaged by pests (bugnibbled and ridden grains) grains in which the germ is discoloured (to brown or black colour) and is not sprouting, and grains overheated during drying. Their total maximum allowed amount is 7%, with a maximum of 0.5% of the overheated. 43 Created by XMLmind XSL-FO Converter. 7. Requirements for cereals and their products (Physical, chemical and functional properties) I. 3. Mottled grains and grains affected with fusariosis. Their total maximum allowed amount is 5%, with a maximum of 1.5% of the ones affected with fusariosis.. 4. Sprouted grains can be present in 4% at most; they are all the grains their radicle or plumule can be seen. 5. At most 3% of miscellaneous impurities (Schwarzbesatz) is allowed, containing what are the noxious and other extraneous seeds (all the non-cereal grains, the limit value for noxious seeds is 0.1%), damaged grains (do not suitable for human and animal consumption due to other reasons, e.g. heat damage, wheat midge with a limit value of 0.05), extraneous matter (all those materials what do not passes through a sieve with apertures of 3.5 mm or passes through it with apertures of 1.0 mm, regardless inorganic or organic character), husks, ergot (maximum 0.05% is allowed), decayed grains and dead insects and fragments of insects. 6. Live pests are cannot be present in the lot 7. Piebald grains which have lost their vitreous aspect (maximum 27%) These limit values above are valid separately; the total percentages of these categories should not exceed 12% in total. The national or other standards and recommendations may define other categories and other limit values (e.g. the MSZ 6383:1998 Hungarian Standard for wheat quality allows only 2% for broken grains and 2% for sprouted grains, do not allow the presence of bedbug stung seeds and the total foreign matter content should not exceed 2%. Before the analysis, the purpose of evaluation should be clearly clarified. The specific weight is the third physical parameter what has to be evaluated during quality control. It is the mass of one hundred liters grain in kilograms. The mass of specially prepared of grains with known volume is measured and corrected by the congeries using an experimental table. This parameter refers to the flour yield, therefore a very simple, chemical free and rapid but informative test is it and it was used widely in the industry formerly. This parameter is influenced by specific gravity of grains, their moisture content, the sizes of grains and their fullness and the surface of hull. The minimum requirement is 73 kg/hl by the intervention regulation of EC while the Hungarian Standard requires higher value for the use in baking industry 3. Chemical properties The chemical composition of wheat is less regulated for qualification, but it is necessary to know the ratios of chemical components and understand their roles in use. In general, the cereals are rich in energy provided by their relatively high carbohydrate contents, and have medium and moderately high protein contents. The chemical composition shows differences in the different physical parts of grain therefore first an overview of physical composition is necessary. The grains of cereals can be divided into three main parts; bran, germ and endosperm. Between the bran and the endosperm there is a narrow, one-cell-wide layer of aleurone cells what should be classified into the endosperm layer by function but it is hard to separate from the bran in general. The bran is about 10% of the total mass of grain with a function of protection of grain from harmful external effects. It can be divided into four external layers of pericarp and two layers of seed coat. The seed coat is an important part, because it impedes the water to pass through into the endosperm and hinders leaving the vapor when the moisture content of grain decrease under a critical value required for germination. It is also important for the milling industry; the moistening of grains makes easier to separate the bran and endosperm part with the aleurone layer. The aleurone layer is about 5 to 7% of the total mass of grain. The germ or embryo is separated from the endosperm by the scutellum, and its main role providing the germination in favourable conditions, therefore containing the genetic information and rapidly utilizable energy content for this process. It is about 4 to 5% of the total mass. The endosperm is the storehouse of the grain; its 80% contribution to the total mass provides nutrients and energy for the processes during storage and for the juvenile plant at the first stages of germination. Anatomically two more important parts of the surface of wheat grain have to be mentioned; the hair on the opposite side of grain to the germ and the crease on the longitudinal opposite side of grain to the germ. They are important from food safety aspects; the external contaminants (especially dust with metal contaminants and microbes and their toxins) easily remain in them, therefore their proper cleaning is necessary. The moisture content of wheat grains is depends on the conditions of harvest; it varies from 12 to 18%. As the high moisture content is favourable for physiological and microbial spoilage, the moisture content of wheat grains has to be decreased below 14.5% in the countries with climatic conditions similar to Hungary for long 44 Created by XMLmind XSL-FO Converter. 7. Requirements for cereals and their products (Physical, chemical and functional properties) I. term (4 to 12 months) storage. Colder climate, shorter storage time or ventilated storehouse may allow storing with higher moisture content. Carbohydrates have the highest amount of substances in the dry matter content of cereals; its average concentration is 70 to 75% in the wheat grains. The starch is present in the highest ratio, its concentration is about 65%. The starch is formulated from amylose (containing α 1-4 bonds between the glucose molecules and forms a linear chain) and amylopectin (containing α 1-4 and α 1-6 bonds simultaneously, resulting branching in the chain). The ratio of amylose and amylopectin is one to three (that is 25% of starch is amylose) and this ratio is similar for the other cereals as well, in the case of corn it is about 28% of amylose of total starch content and this value is 22% for barley, 27% for rye and oat, 18% for rice. In the case of specific cereals this ratio is modified artificially; waxy varieties or hybrids have much lower amylose contents (waxy corn, waxy rice and waxy sorghum have only 1% amylose contents) but corn hybrids are available with high amylose content too (more than 50%) resulting significant changes in the digestibility of starch; the conventional corn hybrids have their starch digestibility about 95%, but the high-amylose corn hybrids have about 60 to 75%. The appearance of starch granules produced from the endosperm part of grains depends on the species of cereal; they can be classified by size (small, medium, large) and shape (sphere, lens-shaped, rectangular or polygonal) of granules making possible the visual differentiation. The cereal species with the highest starch contents are the rice and certain corn types (even higher than 80%) and oat has the lowest one (55 to 60%). The monosaccharide and oligosaccharide content of wheat and other cereals is relatively low; it is about 5% in general during storage. The other part of polysaccharides is the fiber. The fiber content of cereals are ranged from 1 to 4%, includes cellulose, hemicellulose and pentosans. The protein content of cereals is ranged from 7 to 17%, depending on the species, variety and preparation. The wheat has its protein content between 10 and 16% and the lots with higher protein contents are preferred; only use in starch and related industries (sugar products, ethanol) and biscuit making requires low protein content because of the negative correlation between starch and protein contents. From the cereals, in general rice and certain corn varieties and hybrids have low protein contents (from 7 to 10%). From the aspects of use not only the protein content is important considering quality, but its composition. The different cereal species have different amino acid compositions and the production (agronomy) also has influence on it. The increase in protein content result increase in amino acid content, but the increase in the amount of amino acid content result decrease in the biological value of protein – the ratio of essential amino acids decreases. In general, cereals are poor in lysine. The types of proteins present in the cereal grains can be classified chemically and functionally by their solubility. The protein fraction which soluble in water is the albumins, the fraction soluble in dilute salt (NaCl) solution is the globulins, the fraction soluble in a mixture of ethanol and water is the prolamines and the fraction soluble in acidic and alkaline solutions is the glutenin. The protein fractions of specific cereals have their specific names; the albumin fraction of wheat is called leucosin, the globulin fraction is called edestin, the prolamine grous is called gliadin and the glutenins called glutenin. The albumins and globulins forms the structural proteins of grains; their ratio in the total protein content is about 10 to 15% for wheat, 13 to 24% for barley, less than 10% for rice and corn and higher than 80% for oat. The prolamines and glitenins are the storage proteins together and also called gluten proteins. They are insoluble in salty water, but in the case of wheat they can absorb a lot of it (more than threefold of their mass) and the wet gluten forms a three-dimensional protein network what has an unique elasticity and strength against deformation and behavior can be utilized in bread making. Unfortunately, an ever-growing part of the mankind shows allergic reaction to gluten (coeliac disease). The technological properties of wheat flour are strongly influenced by the gliadin-glutenin ratio and composition; their ratio ranges from 1 to 1 to 3 to 1, depending on the variety and the circumstances of growing. In general the higher gliadin content results soft gluten and the higher ratio of glutenin results more strong gluten. The gluten can be evaluated by its amount and quality. The determination of gluten content is based on its characteristic, that is the non-water-soluble but hydrating proteins which forms a three dimensional elastic, but ductile network. Therefore, when the flour is mixed and the formed dough is washed with NaCl solution the soluble components (carbohydrates, lipids, water and salt soluble proteins, fibers, higher ratio of mineral elements) are washed out and only the gluten and minimal amount of other components are remains. The mass of the remained centrifuged gluten in the proportion of the mass of the original flour is the wet gluten content. The dry gluten content is the mass of the gluten dried on 103°C in the proportion of the mass of the original flour. The wet gluten content is ranged from 25 to 24% in general and the dry gluten content is about the third of the weight of the wet gluten. 45 Created by XMLmind XSL-FO Converter. 7. Requirements for cereals and their products (Physical, chemical and functional properties) I. The quality of gluten can be characterized by gluten spreading and gluten index. The evaluation of the gluten spreading uses the washed wet gluten; a sphere of 5 g centrifuged gluten is formed, its dimensions (width and length) are measured. It is left alone for an hour, then its dimensions are measured again and the average difference between the rested and original gluten sphere in mm us the gluten expansiveness. Its value is ranged from 0 to 15 mm generally. The gluten index test also uses the washed and centrifuged gluten; it is measured, centrifuged again using a special sieve when the strong gluten particles remain on the sieve while the soft ones pass through. The mass of the remaining strong gluten part in the proportion of the mass of the washed gluten is the gluten index, therefore the higher value (closer to 100%) means stronger gluten. Although the two tests are similar, there is difference between the results; the value of gluten index depends only the ratio of strong and weak gluten components while the gluten expansiveness is influenced by the proteolytic activity as well. The sedimentation volume is also a protein characteristic quality parameter. Its basis is that some proteins of flour precipitate in acidic solution and the amount of precipitation measured after a specific time refers to protein properties. The higher volume means higher protein content and better protein properties. The widely used type of this test is the Zeleny’s sedimentation volume. Lactic acid solution as acidic agent is added to the wheat flour-water suspension and mixed in the presence of colouring compound for a specific time, then the measuring cylinder is left alone – the sedimentation volume is the volume of suspension after 5 minutes resting. The higher volume means more protein content and better protein properties. The flour quality is low above 30 ml sedimentation volume and this flour cannot be used in the baking industry. The lipid content of wheat is low; it is between 1.5 and 3%. This low value is typical for other cereals too; the corn has a higher value (about 5%) and the oat contains the most from cereals, it is between 6 and 8%. The concentration of mineral elements is also low; 2 – 2.2% is typical for wheat. The distribution of chemical components in the three main parts is hot homogeneous. The endosperm part (without the aleurone layer) has the lowest protein content, the bran with the aluerone layer contains twofold and the germ threefold amounts. Similarly, the endosperm is the poorest in lipid content, the bran contains tenfold and the germ twentyfold amounts; the ash content of bran is twentyfold comparing to the aleurone layer and the same value for germ is tenfold. Similar ratios aresaid for the fiber content. In contrast, the endosperm part contains only starch. This means that the white flour has a significant nutritional disadvantage comparing to the whole grain flours both in protein, fiber, element and vitamin concentrations and energy content. The enzymes are present in the chemical composition in small amounts but their role is significant. The most important ones are the amylases for the winter wheat from the aspects of storage and baking industry. The amylases destruct starch to glucose providing substrate to the respiration. The higher amylase activity refers to higher physiological activity, therefore exposure to spoilage. On the other hand, during baking the amylases provide glucose for the yeast and if the enzymatic starch degradation is too rapid then the growth of yeast will be too rapid and it will be unfavourable from the view of bread structure. In contrast, low amylase activity results succinct and compact bread crumb. The amylase activity is evaluated by the Hagberg’s falling number method mostly. A flour-water suspension in hot water bath is mixed for a minute, then the mixing rod falls in the starch suspension. This falling will be slow due to the fact that boiling water gelatinizes the starch in the suspension, but the amylases start to breakdown it. The higher amylase activity result faster starch degradation and faster decrease of viscosity, therefore the rod will be got down sooner. The falling number is the time in seconds from the beginning of mixing to the arrival of rod to the bottom of tube. 4. Rheological properties The technological analysis of wheat flour is based on the evaluation of the rheological properties of the dough made from it. Many methods are developed, but the Farinograph and Alveograph tests are most widely used ones. The Farinograph test evaluates the dough in dynamic conditions by continuous kneading using two z-arm mixer. The equipment records the resistance of dough against the deformation and displays a diagram (Figure 11.). The curve has upper and lower limit lines due to the attenuation of the arms and a drawn midline what averages the values presented by the upper and lower line. The test does not apply the same ratios of flour and water, but requires a specific resistance to adjust with the addition of water to the same amount of flour. This specific resistance is 500 BU (Brabender Unit) what is an arbitrary unit of viscosity. The more water addition results weaker the less water addition results stronger dough. The amount of water expressed in percentage is the water absorption capacity, what is the amount of water used in bread making. Its value is ranged from 55 to 70%, the higher values are more advantageous. It is hard to add as much water as necessary to reach the 500 BU exactly therefore a small tolerance is allowed; the obtained maximum resistance line has to be within 20 BU from the 500 BU line. 46 Created by XMLmind XSL-FO Converter. 7. Requirements for cereals and their products (Physical, chemical and functional properties) I. 7.1. ábra - Figure 11.: Representative Farinograph diagram The valuation of curve is different in the international and Hungarian qualification system. In the international evaluation the readings are the arrival time (the time when the upper line of curve crosses the maximum resistance line), the peak time (the time when the curve reaches its maximum value), the mixing tolerance index (the difference of resistace values measured in the peak time and 4 minutes after), the departure time (the time when upper line goes above the maximum resistance line), stability (the time between the departure and arrival times) and Farinograph Quality Number (the tenfold of the time expressed in seconds from the beginning of evaluation to the time when the upper line falls 30 BU below the maximum consistency line). In Hungary, the dough development time (the time when the midline reaches its maximum value), stability (the time while the midline and the maximum resistance line are parallel to each other), degree of softening (the distance between the midline and the maximum tolerance line in BU) and the baking value (calculated from the area between the maximum resistance line and the midline) are the evaluated parameters. The latter one is the basis for the Hungarian Qualification, based on this value the sample can be classified as one with improving quality (longlasting stability, minimum softening). milling quality (medium softening) or feed quality. The Alveograph test is a biaxial stretching using constant 2 to 1 ratio of flour and 2.5% NaCl solution, taking into account the moisture content of flour. The dough is mixed, formed, rested then tied down a clamping device and inflated until it gets torn. The equipment draws a curve with the pressure in the dough bubble on the y axis and time on the x axis (Figure 12). The height of the curve is the P value (expressed in mm) characterizes the strength of the dough; this is the maximum pressure to what the dough can resist; the length of the curve is the time from the starting of deformation until the dough gets torn – this value expressed in mm is the L value characterizes the extensibility of dough. Their ratio is the P/L value, the formal quotient of curve and the area under the curve is the W value expressed in 10-4J, the work required for the final deformation of dough. The international recommendations contain reference values for different uses of flour. 7.2. ábra - Figure 12.: Representative Alveograph diagram 47 Created by XMLmind XSL-FO Converter. 7. Requirements for cereals and their products (Physical, chemical and functional properties) I. The Extensigraph is an uniaxial extension. A dough with 500 BU consistency is prepared using flour and 2% NaCl solution in the bowl of the Farinograph, formed to a cylinder, rested for 45 minutes, then its ends are impacted and its middle part is pulled off with a constant speed until the dough breaks. Next, the dough is formed again and the resting and extension is repeated two times (in the 90 and 135 minutes). The three deformations are recorded on three diagrams (Figure 13), there the x axis is the time starting from the deformation, characterizes the extensibility of dough and the y axis is the resistance of dough against the extension. The quality parameters are the maximum resistance (the highest resistance value of the curve), the resistance to extension5cm (the resistance of dough measured in the 5th cm of the x axis), the extensibility (the length of the curve) and the deformation work (the area under the curve). 7.3. ábra - Figure 13.: Representative Extensigraph diagram 5. Other quality parameters 48 Created by XMLmind XSL-FO Converter. 7. Requirements for cereals and their products (Physical, chemical and functional properties) I. In special cases other quality parameters may necessary to evaluate, for example microbial state (fungi, especially molds), toxin content, heavy metal content or pesticide residue and other chemical content. The limit values for these parameters are can be found in international legal sources. 6. Qualification of wheat The limit values for the quality parameters of winter wheat are different in the standards and recommendations of different countries and purposes of use. The requirements can be acceptance of classification. The intervention quality requirements of the European Commission are acceptance requirements; all the lots have to fulfil its parameters to be accepted. Its limit values were previously presented, beside them it contains limit value for the moisture content (maximum 14.5%), protein content (minimum 10.5%), specific weight (minimum 73kg/hl), falling number (minimum 220 s), Zeleny’s sedimentation volume (minimum 22 ml, but if the result is between 22 and 29 ml then dough machinability test is required to perform). In contrast, the Hungarian standard lists classification requirements (Table 2). Based on the readings, the lots are classified into one of the quality groups: improving, milling I, II and III or feed quality with attention to the requirements of baking industry. 7.1. táblázat - Table 2.: Detailed quality requirements on wheat (MSZ 6383:1998) Quality characteristics Common wheat Improving wheat Durum wheat Milling wheat I. II. III. I. II. 78 75 Test weight, at least, kg/hl 78 76 72 Moisture content, at most, % (m/m) 14.5 14.5 14.5 14.5 14.5 Foreign material content, at most, % (m/m) 2.0 2.0 2.0 2.0 2.0 - harmful foreign material at most, % (m/m) 0.5 0.5 0.5 0.5 0.5 - light foreign material, at most, % (m/m) 0.5 0.5 0.5 0.5 0.5 - Broken seeds, at most, % (m/m) 2.0 2.0 6.0 2.0 2.0 - Germinated seeds, at most, % (m/m) 2.0 2.0 5.0 2.0 2.0 - Rye, at most, % (m/m) 2.0 2.0 3.0 – – - Deminished value seeds, at most, % (m/m) 2.0 2.0 2.0 3.0 3.0 - Colored surface seeds, at most, % (m/m) – – – 3.0 8.0 - Bedbug stung seeds, pc % – 2 4 2 2 Common wheat seeds, at most, % (m/m) – – – 4.0 10 Hard wheat seeds, at least, pc % – – – 60 30 Details: Permitted above foreign material content: 49 Created by XMLmind XSL-FO Converter. 7. Requirements for cereals and their products (Physical, chemical and functional properties) I. Baking quality, at least*, quality group A B1 B2 – – – Wet gluten content, at least, % (m/m) 34 30 28 26 32 30 Wet gluten expansiveness, mm/hour 2-5 3-8 3-8 – 2-5 2-5 Hagberg’s falling number, at least, seconds 300 250 230 220 300 250 Crude protein content, at least, % 12.5 12.5 12.0 11.5 12.5 12.0 Zeleny sedimentation index, at least, ml 35 35 30 20 – – Yellow pigment content**, at least, mg/kg – – – – 5.0 3.5 Pests and their remains Cannot contain * The lower limit for the „A” quality group is 70, for „B1” quality group is 55 and for „B2” quality group is 45. ** In dry matter 50 Created by XMLmind XSL-FO Converter. 8. fejezet - 8. Requirements for cereals and their products (Physical, chemical and functional properties) II. 1. Quality and qualification of rye Rye is the second bread cereal beside wheat. Its chemical composition is similar to that of wheat in the main parameters (protein, carbohydrate, starch, lipid and ash content), but the most important difference is that although rye has gluten proteins its gluten proteins do not forms that three-dimensional structure what the one of wheat does. It is caused by the present mucilages and gums hinder the gluten proteins to aggregate. In contrast, the bread made from rye has similar structure to what made from wheat but this case the carbohydrates has significant role in the formation of crumb structure. The starch of rye is more sensitive, contains more broken chains than that of wheat, therefore the amylases degrade it easier. The starting temperature of gelatinization is lower for the starch of rye and the water permeability of its bran is higher to that of wheat; these all together make more difficult to use in the baking industry. The quality requirements of rye by the MSZ 6342:1984 Hungarian Standard defines for both food and feed use. The analysed parameters are the specific weight with a limit value 71 kg/hl for food use and 67 kg/hl for feed use. The allowed rate of impurities is 2% of which noxious seed can be maximum 0.5% and ergot maximum 0.2%. The maximum concentrations of light impurities, broken grains and sprouted grains are 0.5%, 3% and 2% for food use and 0.5%, 10% and 5% for feed use, respectively. The required moisture content 14.5% at most. The parameters to control for food use list other requirements what characterizes quality from the aspects of baking use. These are connecting to the properties of starch from the previously detailed aspects. Limit values for falling number are present; it has to be between 150 and 250 s. The second specific evaluation is the Amylograph test. It is a rotational viscometer-like rheological test; a thin suspension is made from flour and water, mixed and places into a special bowl with vertical sticks. This bowl is rotating during the analysis and its temperature is gradually increased by a heating unit; the increase of temperature is 1.5°C in every minutes. Sticks of an upper stable unit sink into the suspension and while the lower unit rotates the suspension exerts a force on the stick due to its viscosity. The analysis starts on 25°C and an unit records the force can be measured on the sticks. This viscosity is low as long as the temperature reaches the gelatinization temperature, then the gelatinized starch rapidly and significantly increases the viscosity. After reaching a maximum point the viscosity starts to breakdown due to the amylase activity and at the end of test the saccharification is complete and the viscosity is low again. The readings of Amylograph test are the starting temperature of gelatinization (°C), the temperature of maximum viscosity (°C) and the Amylograph value, what is the maximum viscosity (BU, Brabender Unit). The standard requires an Amylograph value between 300 and 900 BE for baking use. 2. Quality and qualification of corn Corn is the cereal with second highest harvesting area in the world, comes after the wheat. Its high cropping area is because of its high yields, rapid genetic improvement, fully mechanization is resolved and several fields of uses are developed. The most of volumes had been used for feed and forage, but the development of industrial kind of uses became dominant in the last 30 years. The industrial use of corn os based on its high starch content; starch is the primary main product but the further processing produces starch derivates, sugar products (corn syrup) and can be used in the fermentation industry, especially for ethanol production for food, industrial and energy uses. The by-products of the industrial processing ate also important; the corn oil is used for human consumption and the Dried Distillers Grains with Solubles (DDGS) for animal nutrition. The traditional food products of corn are the ones from milling industry (cornmeal, grist and flour), popcorn and sweet corn. The corn meets about 60 to70% of energy demand of livestock and about 40% of protein demand. It does not contain any chemical component what might limit its use in nutrition (anti-nutritive compound, alkaloid, etc.). The carbohydrate content of some of its types is nearly the highest of the cereals, hybrids bread for starch industry contains more than 80% in the dry matter content. The hybrids used for livestock feeding have lower starch contents (about 75%) while the popcorn and sweetcorn varieties have less than 70%. Their protein contents are relatively low; it ranges from 5 to 13%. The amino acid composition of proteins are 51 Created by XMLmind XSL-FO Converter. 8. Requirements for cereals and their products (Physical, chemical and functional properties) II. disadvantageous, contains less essential amino acids (lysine, methionine, cysteine) than wheat or barley. Significant is its lipid content; the corn germ oil is high comparing to other cereals (expect oat), it is about 4 to 5%, rich in unsaturated fatty acids. The ash and fiber content is lower than the ones of the other cereals. Due to the wide range of using and processing possibilities there are a lot of standards and recommendations are available for corn. The general standards are the trading ones. In Europe the UNECE deals with the sweet corn only. The intervention regulation of the European Commission declared requirements on corn, but this product was removed from the intervention a few years ago. The limit values were declared for the purity and moisture content only. The United States Department of Agriculture separates five groups in qualification (from U.S. No. 1. to No. 5.) based on the test weight (the requirement for meet the U.S. No. 1. grade is 72 kg/hl and 59.2 kg/hl is the limit for the U.S. No. 5. grade), damaged grains (the requirement for meet the U.S. No. 1. grade is 3% with a maximum of 0.1% of heat damaged ones, and 15% is the limit for the U.S. No. 5. grade with a maximum of 3% of heat damaged ones) and broken grains and foreign material (maximum 2% for U.S. No. 1. grade and 7% U.S. No. 5. grade). The lots do not meet the requirements for the five grades or contain stones or musty or have foreign odor are called U.S. sample grade corn. The stock exchanges define their own quality demands.. The requirements Budapest Stock Exchange say that the lots offered for trade must be healthy, dry, shelled, free from living pestiferouses and mouldiness. Its moisture content has to be 14.5% maximum, the allowed ratio of impurities is 2%, in which harmful impurity is maximum 0.5%. The allowed maximal amount of materials by other impurities is 2% for sprouted grains, 3% for heat damaged kernels, 8% for broken grains and 2% for small fragmentaries (2.5 mm; 2.5-4.5 mm). The Hungarian Standard defines the requirements in three standards for corn. The MSZ 12540-1998 contains the limit values of corn for forage and lists general requirements (purity and impurities), moisture content and chemical components important for nutrition (protein content, fat content, fiber content and ash content) (Table 3.). 8.1. táblázat - Table 3.: Requirements on corn grains for forage (MSZ 12540-1998) Parameter dried wet corn Moisture content, %, max 14.5 Purity, %, min 98.0 Protein content, %, min 7.6 Fat content, %, min 2.9 Fiber content, %, max 3.4 Ash content, max 1.9 Impurity, %, max 2.0 of which harmful impurity, %, max 0.5 Allowed amount of materials sprouted grains 2.0 3.0 by other impurities, %, max* heat damaged grains 3.0 5.0 broken grains 8.0 8.0 small fragmentaries 2.0 3.0 52 Created by XMLmind XSL-FO Converter. 8. Requirements for cereals and their products (Physical, chemical and functional properties) II. *optional requirements, contractants may modify The official methods for the determination of the chemical parameters listed above are classic ones (e.g. evaporation method for moisture content, Kjeldahl method for protein content, extraction method for lipid content, ashing method for ash content and the destruction with sulfuric acid and potassium hydroxide for the fiber content. The demands (rapid price determination, rapid decision on acceptance) emphasize the role of rapid analytical methods. The NIR/NIT devices are frequent in the practice. These devices can be used for the rapid determination of moisture, protein and ash content with good accuracies, but the estimation of starch and fiber content is more uncertain. The electric conductivity measuring devices can be used for measuring water content reliably, but the accuracies of these methods strongly depend on the calibration; the professional calibration and frequent control and recalibration can guarantee the proper result. The MSZ 6180-1980 summarizes the requirements on corn used in the milling industry based on the general requirements (purity and impurities), moisture content and floating number (Table 4.). The floating number is correlates to the grits yield. The determination is simple and rapid; grains have to be put into sodium nitrate solution with 1.25 g/cm3 concentration and they separate by density. The higher density results lower number of floating grains. The result is the floating number (without dimension) what the ratio of floating grains is. 8.2. táblázat - Table 4.: Quality requirements for corn for food use (MSZ 6180-80) Parameter Requirement Moisture content, max 15,0% Purity, min 98,0% Impurity, max 2,0% In which harmful impurity, max 0,5% firefanged grain, max 1,0% Allowed amount of materials by other impurities, max sprouted grains 1,0% small fragmentaries (fall through 6 mm sieve 5,0% crackled grains 15,0% Floating number - for fractionation max 50 - for flaking min 50 The third group of requirements defines demands for the sweet corn; it prescribes demands on purity, moisture content, enzyme activity (peroxidase test) and microbiology (Table 5.). 8.3. táblázat - Table 5.: Quality requirements for sweet corn (Győri-Győriné Mile, 2011) Parameter Requirement 53 Created by XMLmind XSL-FO Converter. 8. Requirements for cereals and their products (Physical, chemical and functional properties) II. Physical tests moisture content 69.5 % empty skin max 1% teared grain max 1% broken, badly cut grain max 4% discoloured grains max 1% Microbiological tests aerob microbes max 5 105 total coliforms max 3 103 thermotolerant coliforms (44°C) max 15 E. coli 0 Clostridium perfringens max 10 Staphilococcus aureus max 10 yeast-fungus max 1000 hyphomycetes max 500 Salmonella (in 25g) 0 Listeria monocytogenes (in 10g) 0 Peroxydase test negative The estimation of fermentable product is a key question of corn quality control for the bioethanol industry. References found that there is no direct connection between the starch content and potential ethanol yield of corn samples because the composition of starch influences the realizable ethanol yield. From 87 to 94% of the starch content can be transformed to ethanol in general and the extractable starch content shows better correlation to ethanol yield. The proper result can be achieved by fermentation tests modelling the production process, but the NIR spectral analysis is also found to be suitable for prediction. Due to the increasing role of biofuel production it is important to mention the quality parameters of DDGS, even more the third part of corn processed in ethanol industry nowadays. The DDGS is the dried remaining material after the distillation of ethanol and contains the proteins, fibers and other non-fermentable carbohydrates, lipids and minerals of corn, the remaining of the yeast cells, what are rich in proteins, and the other products of them. Its chemical composition can be characterized by high protein content (about 30-32%) of which 50% is rumen undegradable protein, high methionine content, 12% fat content, high fiber content (12% ADF, 25% NDF) The requirements of Codex Alimentarius on the quality of milling products of corn list general demands on appearance, sensory properties and physical and chemical properties. The moisture content should not exceed 14% and the fat content can be maximum 2.3% for flour and 2.0% for grist and cornmeal due to the exposure to 54 Created by XMLmind XSL-FO Converter. 8. Requirements for cereals and their products (Physical, chemical and functional properties) II. risk of rancidity. The allowed maximum value for acid number is 5 and particle size distribution demands are also included based on the character of products. 3. Quality and qualification of rice The rice is the crop harvested with the highest worldwide production beside corn and wheat, but in contrast to the other cereals about 95% of the total production is used for human consumption therefore the importance of industrial use is significantly lower. The non-food uses are the starch and cosmetics production. The processing of rough rice or paddy rice starts with the removal of chaff; its product is the brown rice. The following step is the removal of bran and germ to different degrees resulting milled rice or white rice and it can be polished. Due to the differences in the chemical composition of different grain parts this processing way significantly influences the chemical composition of the different products. In general the high energy content is the main characteristic of its chemical composition. It is provided by its high carbohydrate, especially starch content. The rough rice has 64 to 73% carbohydrate content and 1.5 to 2.3% fat content, high (7 to 10%) fiber content and also high ash content (3 to 5%), but its protein content is one of the lowest of the cereals, it is between 5.5 to 8%. The hulling decreases the nutritional value of the grains; although the protein content of brown rice is similar, its fiber content decreases to tenfold, ash content to threefold but the starch content increases by 10%. The milling makes these values worse; the protein content of milled rice is between 5.5 and 7%, carbohydrate content is between 77 and 89%, ash content is lower than 0.8% and fat content is below 0.5%, therefore it can be concluded that the nutritionally important chemical compounds are in the bran and hull of rice, however the rice has the highest protein value and its starch is the most digestible of cereals. There are several types and varieties of rice. They can be classified by the shape of grain; it can be slim when the ratio of length and with is higher than 3, medium, when this ratio is between 2 and 3, semi spherical when this ratio is between 1 and 2 and round if it is less than 1. This profile index can be applied on all products from brown to polished rice. The industrial use prefers the round and semi spherical types while the consumers like the long and middle grains more. It is also expected that the grains swell good and do not stick together. The amylograph properties of rice refer to the stickiness by the evaluation of starch gelatinization. The requirements on paddy rice of the European Commission prescribe a maximum value of 14.5% for moisture content. The lots have to be free of odor and should not contain live insects. The maximum percentages of different impurities are determined for chalky, red stained, spotted and stained, amber, yellow grains, miscellaneous impurities and other varieties than the main one (Table 6.). 8.4. táblázat - Table 6.: Maximum percentage of miscellaneous impurities in paddy rice (EC No 489/2005 of 29 March 2005) Grain defects Round-grain rice Medium and grain A long Long grain B Chalky grains 6 4 4 Grains striated with red 10 5 5 Spotted and stained grains 4 2.75 2.75 Amber grains 1 0.50 0.50 Yellow grains 0.175 0.175 0.175 Miscellaneous impurities 1 1 1 Rice grains of other varieties 5 5 5 55 Created by XMLmind XSL-FO Converter. 8. Requirements for cereals and their products (Physical, chemical and functional properties) II. In the requirements of Codex Alimentarius Hungaricus on the quality parameters of rice products defines three groups. The first one is the brown rice, the two others are white rice with different quality level; the type A contains less fragmentary and impurities and higher values are allowed for the type B. Requirements are listed for moisture content (it has to be less than 15%) and purity. In general, the maximum allowed foreign matter content is 0.2% for type A rice and 0.3% for brown and type B rice and 0.1 and 0.2% can be harmful one, respectively. For brown rice the maximum allowed rate of polished and broken grains is 3-3% each and it is 5% for the ratio of grains with red silver skin and immature grains. Limit values are listed for the amounts of discoloured and plaster grains, coarse (larger than 2.4 mm) and small (smaller than 2.4 mm) fragmentary and the grains with red stripe. Rice grains have two longitudinal creases; during milling the brain is removed and when the milling is not completed, the brain remained in these creases. These are the red stripes mentioned in the requirements. 4. Quality and qualification of barley The barley is a traditional feed crop and the food processing uses it for malting mainly, but the milling industry also produces pearl and husked barley and flakes. The barley species show great variability. The basic classification is by their sowing period (spring or winter) and the number of rows of grains along the rachis (tow, four or six). Generally the six row varieties has higher protein content in comparison to the two row varieties therefore the variety type determines the use; six row varieties are used in animal husbandry and six row varieties for malt production. The protein and fiber content of barley have significant role in its nutritional value. The protein content of barley is between 10 and 17%. the starch content is varied from 65 to 70%. The crude fiber content of winter barley is about 6%, the one of spring barley is about 4.5% and the one of nude barley is about 1.3%. According to these values, the dietary fiber content of barley is higher than that of the common cereals; it is between 11 and 34% what can be utilized well in the pig rearing due to the advantageous effect of high fiber content on the fat content of sow’s milk. The β-glucan content of barley is also a projecting value. The MSZ 6372-78 Hungarian standard on forage barley quality requires 14.5% moisture content and 63 kg/ha specific weight. Its other demands are about the purity of lot; the maximum allowed rate of impurities is 2% (not counting wheat, rye and oat); 0.5% limit value is prescribed for light and harmful impurities each. The sum of rye and oat can be 10% at most in the lot and the maximum amount of broken grains is 5% (Table 7.). 8.5. táblázat - Table 7.: Quality requirements for barley for forage use (MSZ 6372-78) Parameter Requirement Specific weight, min 63 kg/hl Impurity, max* 2,0% in which harmful impurity, max 0,5% Light impurity, max 0,5% Allowed rate of broken grains, max 5,0% Sum of rye and oat, max 10% Moisture content, max 14,5% * Wheat is not imputiry in barley The most important requirement of malting use is the good germination ability. The malting is the germination of grains actually and the malt is the grain after the break of sprout from it. Therefore, the MSZ-08 1326-79 Hungarian standard summarizes the quality requirements for malting barley prescribe 95% germination ability on basis. The grains ideal for beer production have to be matured, full, light yellow coloured and without other 56 Created by XMLmind XSL-FO Converter. 8. Requirements for cereals and their products (Physical, chemical and functional properties) II. colouring. The standard lists these parameters in details; the specific weight has to be higher than 68 kg/hl, longer than 6 mm and wider than 2.5 mm. Its bran has to be thin and moistened homogeneously. The protein content has to be low; partly because the low protein content refers to the high starch content and partly because the protein content of grains influence the taste and durability of beer foam. The basis value for protein content is 11.5%. Other requirements are about the purity (98% at least) and moisture content (14.5% at most) (Table 8.). 8.6. táblázat - Table 8.: Quality requirements for malting barley (MSZ-08 1326-79) Parameter base limit Specific weight, kg/hl 68,0 min 65,0 Purity, % 98,0 min 96,0 Impurity, % 2,0 max 4,0 in which valuable impurity, % 1,5 max 3,0 valueless impurity, % 0,5 max 1,0 harmful impurity 0,2 max 0,5 Grains with full value (2.5 mm sieve), % 75,0 min 70,0 screenings in 2.2 mm sieve, % 4,0 max 5,0 germinating ability, % 95,0 min 90,0 Gradedness Moisture content, % 14,5 Protein content in dry matter content 11,5 max 12,5 5. Quality and qualification of oat Despite the human consumption of oat has several benefits, it is mainly used as feed grain, especially in the diet of horses and sows, and in some cases of cattle. The human consumption is common in the USA, England and the Scandinavian countries as breakfast cereal (rolled oats, oatmeal). The oat shows the highest differences to the other cereals in chemical composition. Its protein content is about 10 to 13%, a little less than the one of wheat, but the concentrations of essential amino acids are higher (about 50% of amino acids are essential) and the nude oat varieties have 20 to 30% higher protein content. It has significantly lower starch content in comparison to the other cereals, it is about 55% in general. The oar is rich in lipids, this commonly grown cereal has the highest lipid content with a value from 5 to 7% and this lipid content is rich in essential fatty acids. The oat has high fiber content also ranging from 8 to 12% what is an outstanding value of cereals containing β glucan, a water soluble polysaccharide behaves as dietary fiber during consumption having several advantageous health effects. The quality oriented breeding programs for human consumption aim to increase its protein and β glucan contents, increase the rate of starch gelatinization, but decrease the oil content. In contrast, the breeding direction is to increase the oil and fat contents with the decrease of β glucan content. The quality requirements for oat are not focusing on the chemical composition. The purity of the lot has to be 98% at least and maximum 0.5% is allowed for the amount of noxious seeds and the same ratio for the light impurities. The presence of wheat, barley and rye is allowed to a maximum 10% concentration. The moisture 57 Created by XMLmind XSL-FO Converter. 8. Requirements for cereals and their products (Physical, chemical and functional properties) II. content has to 14.5% at most and the minimum specific weight has to be higher than 22 kg/hl by the requirements of MSZ 6326-85 Hungarian Standard. The MSZ 6326:1996 standard completes it with requirements on protein content (10.65%) and starch value (54.34 kg/100 kg). It does not make special claims regarding food use, but several countries requires higher specific weight (e.g. 51 kg/hl is the limit value for premium quality in Australia) but similar values for moisture content, impurity and screenings. 6. Quality and qualification of grain sorghum and millet The sorghum and millet has relatively low importance in Hungary comparing it to other cereals. Their chemical composition is similar to those of other cereals (12 to 14% protein content, 65 to 70% starch content) with a less higher fiber contents (the fiber content of millet may reach 9%) and millet has the highest ash content of cereals. They are used for forage, but also had role in human consumption (mush and bread, mixed with wheat flour) and nowadays the puffed millet is a gluten free cereal product providing the nutritional benefits of millet combined with the advantages of puffing technology. The properties of grain sorghum are similar to those of corn, but the digestibility of its proteins is worse. The requirements on millet and sorghum is similar to the other forage cereals, requirements on purity and moisture content are defined. 58 Created by XMLmind XSL-FO Converter. 9. fejezet - 9. Requirements for industrial crops I. (sugar beet and oil plants) 1. Chemical composition and qualification of sugar beet The main raw material for sugar production is the tuber of sugar beet in the industrialized countries and had 20% share of the total processing. The other 80% derives from the processing of sugar cane cultivated in developing countries with tropical climate. Sugar beet root has slightly higher sugar content than sugar cane (17% compared with 13%), and the extraction rates are similarly depends on the varieties; 40 to 80% for sugar beet and 30 to 100% for sugar cane. The industrially used part of sugar beet is its root (tuber) what stores sugars after the first year of vegetation period and helps begin the development in its second year. Therefore the sugar content in the tuber decreases continuously, so the harvest, transport and processing have to be organized carefully and foresight. The tuber has four parts, crown (epicotyl), neck (hypocotyl), root (radix) and tail (cauda) (Figure 14.). The crown is about 6 to 15% of the mass of tuber and has relatively low sugar content, therefore it is cut down with the leaves and used as forage for cattle or for silage. The neck is that part of tuber what is between the crown and root and free from leaves and rootlets. The sugar contents of these parts are below 16-17%. The root gives 75 to 85% of the mass of tuber and has the highest sugar content (its concentration is above 17% and moving to the root tip it continuously decrease. The tail is the part of tuber what has its diameter less than 1 cm. Usually, the sugar content is less than 14% in the tail, so the delivered tuber should not contain it. 9.1. ábra - Figure 14.: Parts of sugarbeet root and their sugar content The physical attributes of the roots allow classifying them by their slenderness ratio and shape index. The slenderness ratio is the quotient of the length and the highest diameter of root and when this quotient is less than 1.5 the root is found to be squat, when it is between 2 and 2.25 it is long and above 2.25 it is very long. The shape index is the quotient of the diameter of root, measured on the half-length and the highest diameter. When value of this index is about 0.50 the root is thick, when it is about 0.55 the root is spool and when it is about 0.65 the root is slim. The water content of root is about 76% and the dry matter is 24%. The dry matter can be separated to insoluble and soluble parts; the ratio is insoluble dry matter components (mainly fiber) is about 4.5 to 5%, the value for 59 Created by XMLmind XSL-FO Converter. 9. Requirements for industrial crops I. (sugar beet and oil plants) the soluble ones is 19 to 19.5%. The compound in it with the highest concentration is the sugar; sucrose is about 75% of the dry matter content (17% on fresh matter base). Beside it, dissolved non sugar compounds are present with about 2.5% concentration, with about 0.5% ash content and 2% organic matter content in it. The organic matters can be classified into colloids and crystalloids and some of the latter one has adverse effect on the extraction of sucrose. There are several components hinder the obtaining of maximum sugar yield. The invert sugar and raffinose content inhibit the crystallization of sugar. The amides and betaine also cannot be separated from the dissolved glucose, they accompanies it through the whole technology and retain a part of sucrose in the molasses. The amount of nitrogen containing components which have adverse effect on the processing also called α-amino nitrogen content and characterized by the “blue number”, referring to its analysis. The potassium and sodium from the mineral elements also decreases the crystallized sugar yield. The quality parameters of sugar beet can be classified into primary ones what can be determined directly and secondary ones what can be calculated using primary ones. On the other hand, physical and chemical quality parameters can be considered. The primary physical quality parameters of sugar beet are the followings: • average root mass and its distribution: the required mass for the tubers is between 0.5 and 1 kg. The smaller roots are woody ones, the larger ones are perishable and poorly storable. • impurities: the impurities to be considered are the organic contamination (maximum 0.2%), root fragmentary (1.3%), crown and leaves remained on the root (5%) and soil (maximum 15%) • damages of root: maximum 5% withered, 1% moldy and 15% of seriously damaged roots are allowed in the lot • ratio of branched roots: branching may significantly decreases sugar content • elasticity modulus: refers to the turgor status, elasticity and plasticity with kg/cm2 dimension. As the modulus decrease, the turgor status of beet can be found to be fresh, slightly withered, withered and strongly withered and the quality group is rigid, elastic, soft and very soft. • resistance against cutting: a rheological measurement to quantify the work is required to cut across an unit of surface using specific blade. Generally, it is influenced by withering status and the fiber content of root. The roots can be classified into soft, normal and woody groups. • diffusion constant of sucrose: quantify the resistance of root against the diffusion of sucrose. The primary physical quality parameters of sugar beet are the followings: • dry matter content • K, Na, Ca, Mg contents: the concentrations of different elements can be determined or the ash content can be characterized by the conductometric ash content. • α-amino nitrogen content (blue number): the α-amino nitrogen colours the copper nitrate solution to blue and its intensity measured by photometer refers to the amount of adverse nitrogen compounds • sucrose content: its rapid determination can be performed by polarimeter • invert sugar content: can be measured by the determination of glucose and fructose concentrations. Industrially UV fluorescent photometry is used: the sugars of heated sample react with added benzamedine resulting fluorescence. • raffinose content • activity of invertase The secondary quality parameters of sugar beet are: • purity – rate of dry matter and sucrose in the root, juice and molasses. The purity ratio of juice is the quotient of the sugar content, determined by polarimeter and dry matter content, determined by refractometer. 60 Created by XMLmind XSL-FO Converter. 9. Requirements for industrial crops I. (sugar beet and oil plants) • extractable sugar content: it can be calculated by the Reinfeld’s formula: Extractable sugar content=SC0.343(K+Na)+0.094N+0.29 where SC sugar content, determined by polarimeter, % K and Na are meq K2O / 100 g root and meq Na2O / 100 g root; N is meq N / 100 g root • alkalinity coefficient: if the alkalinity coefficient of juice is about 2.1 and 2.2 then its K and Na content is sufficient to neutralize the anions and organic acids formed during juice purifying. If it is lower than agents increase the alkalinity has to be used resulting increase in the sugar content of molasses. The qualification of sugar beet in the industry can be performed using automated analyser line. This including the measurement of truck for the determination of gross weight, and then an autosampler take a sample. This sample is forwarded to the cleaner where the roots are washed, the impurities and surface contaminations are removed and the net mass can be measured. Before and after the cleaning the roots are visually examined and the appearance and health status of roots can be determined. Next, the roots are shredded and juice is obtained for the chemical analysis; the sugar content using polarimeter, the blue number and Na and K concentrations using photometer. Based on these reading the extractable sugar content can be determined and the total amount of crystallized sugar from the cargo can be calculated as the reference for the price determination. 2. Chemical composition and quality of oil seeds The plants suitable for oil production are those ones what have higher oil contents than 40%, in general. On the other hand, only those plant parts can be used for oil production economically in which its concentration is higher than 20%. The palm is the plant which has the highest role in the oil production of world; it gives about one-third part of it. The second one with close to 30% role is the soybean; however, its seed does not meet the requirement in the first definition. The ratio of rapeseed is about 15% with an increasing increase due to biodiesel production. The ratio of sunflower seed is below 10% and the ratios of other raw materials (olive, cottonseed, coconut, peanut and others) are below 5%. The requirements on the oil crops are quantitative and qualitative. The qualitative demands are based on the possible uses of products; it can be food use when the primary product is vegetable oil and it can be processed further to margarine, substitutes of cheese and milk products, and different kinds of food additives. The second main way of use is the industrial use; vegetable oils are traditional raw materials of different kinds of chemicals, paints, varnishes, cosmetics and plastic industry. The third way is the energy use; the increasing demand on the biofuels started paying attention to the using of vegetable oils in natural or industrially transformed way. Besides, the seeds feed directly with animals are energy rich fodders, and the byproducts of oil processing (seed meal, oil cake) are rich in protein, therefore they can complement the cereal based animal nutrition. Sunflower, soybean and rapeseed are the most important sources of vegetable oils in the continental region. The seeds of these plants are used in processing and the same issues have to be considered during their storage than the ones for the storage of cereals. To decrease the physiological losses and avoiding the warming of stored lots what may be favourable for chemical and microbiological spoilage the moisture content has a great importance. As the sunflower seed and rapeseed have oil contents higher than 40% significant part of seed has hydrophobic character, therefore the water content concentrates into those parts of seeds where the oil content is lower, especially in the case of sunflower, what is the plant which concentrates seed water content in the shell. Therefore, the moisture contents of these seeds have to be kept low so the one with the highest value should not be higher than 14%. For example, the moisture content of sunflower seeds should not be higher than 9% but the varieties with higher oil content have to be dried more. The oil contents of these seeds usually higher than 40%. The sunflower varieties have their oil contents between 30 to 55%, the varieties with the lower values are suitable for hulled raw consumption. The demand of industrial use is 44% oil content at least. The rapeseed has slightly lower oil content it is between 30 and 45%. The soybean has 16 to 25% oil content in the seed, therefore it is not an ideal raw material, but its role in the animal nutrition due its protein content and the fact that the preparation for forage use starts with the removal of oil content gives high importance for this plant. The oil content of palm seed is about 50%, this value for olive is 40 to 60%, for peanuts 40 to 50%, for cottonseed 30 to 35%. 61 Created by XMLmind XSL-FO Converter. 9. Requirements for industrial crops I. (sugar beet and oil plants) The possible use of oil is depends on its composition. The vegetable originated lipids are liquid in room temperature (except the palm fat) and contain unsaturated fatty acids in high concentrations. The dominant fatty acids in vegetable oils are the oleic acid, linoleic acid, linolenic acid, palmitic acid, stearic acid and eruric acid. When the different vegetable oils are classified based on the dominant fatty acid five groups can be separated by practical consideration (Table 9.): 9.1. táblázat - Table 9.: Classification of plant oils by the main fatty acid (Győri, 1995) 1. group: Oleic acid group Plants with non drying oils (Iodine number: 80-110) Olive oil 2. group: Linoleic acid group Plants with semi drying oils (Iodine number: 100-150) Corn germ oil, soybean oil, sunflowerseed oil 3. group: Linolinic acid group Plants with drying oils (Iodine number: 140-210) Linseed oil 4. group: Erucic acid group Plants with non or slightly drying oils with high erucic acid content (Iodine number: 90-110) Rapeseed oil 5. group: Oxoacid group Plants with non drying oils with high oxoacid content (Iodine number: 80-90) Ricinus The ratios of fatty acids in the different vegetable oils are different; e.g. the linoliec acid content of sunflower seed oil is about 60 to 70% and it is containing about 25% oleic acid. Due to industrial request, in the latest years sunflower hybrids with high oleic acid content in their seeds are also appeared; their dominant fatty acid is the oleic acid with about 80 to 90% ratio. It has better quality characteristics for frying (higher stability) and fits for the demands of bioethanol industry more. The soybean oil also has high linoleic acid concentration (50 to 60%), but the ratio of oleic acid is also significant (about 25%) and the ratios of palmitic acid and linolenic acid are about 10% each. The olive oil has 75 to 80% oleic acid, 10% palmitic acid and 8% linoleic acid contents on average. The oil of common rapeseed contains nearly 50% erucic acid but, due to the disadvantageous health effects of erucic acid, breeding activity aimed to decrease its concentration. As a result the erucic acid contents of newly developed varieties decreased and the ratio of oleic acid increased and nowadays varieties what contains only traces of erucic acid are available for production. The typical parameters for the characterization of vegetable oils are the iodine value, acid value, saponification value, unsaponifiable matter and peroxide value. The unsaponifiable matter is that fraction of oils which cannot be saponified by caustic alkali, but is soluble in ordinary fat solvent. The roles of other values were discussed in Chapter 4. The protein content of seeds of oil plants are also important, as this compound is present with the second highest concentration in the raw materials (the average protein content of sunflower seed and rapeseed is 15 to 25%, this value for soybean is 35 to 40% and about 35 for cottonseed) and the byproducts, the oil cakes and seed mails, have about 45 to 50% protein content. Not only the amount of proteins is significant but its composition is also important; the protein of soybean has the highest biological value from oilseeds, but the protein composition of sunflower seed is also advantageous; 70 to 85% of its proteins are water soluble, containing high amounts of glutamic acid, aspartic acid, arginine, leucine and proline. The vegetable oils also contains micro components, e.g. sterols, tocopherol, carotenoids, vitamins, what increase their nutritional importance. 3. Qualification of oil seeds 62 Created by XMLmind XSL-FO Converter. 9. Requirements for industrial crops I. (sugar beet and oil plants) The requirements on the quality of sunflower seeds can be classified into general, physical and chemical groups by the terms of MSZ 6368:1998 Hungarian Standard. The general requirements list that the seeds in the lot have to be matured, developed, winnowed, healthy, dried and its odor have to be natural. The colour of fruit in the shell should be light gray. The achene should not be damp, moldy, musty, rancid, warmed up, chewed, rotten or should not have foreign smell, but a slight mustiness for the shell is allowed. The lot should not contain living harmful insects. When the lot contains achene with black shell colour, 3% is the allowed ratio for the seeds with different shell colour. The physical requirements list demands on purity. The maximum allowed rate of impurities is 2%, but the different types of sunflower seeds should be ignored during the determination of purity. In this category, the concentration of harmful impurities is 0.5% at most. Limit values are also declared for the seeds with reduced value, in this the maximum ratio of milled (naked) seeds should not been exceed 10%, the rate of broken or damaged achene can be 3% at most and the ratio of achene containing not natural-coloured fruits must be lower than 2%. The chemical parameters provided for sunflower seeds are the moisture content (it should be less than 9%), the oil content (to must be higher than 44%) and the free fatty acid content is not more than 2%. Negative deviation in these nutritive values serves as the basis of depreciation by the contracting parties. Based on the general direction of Budapest Stock Exchange, the basis price is valid for the lots meet the previously listed requirements, differences are basis for specified allowances. 1% difference in oil content 1.5% difference in price for both negative and positive direction. The moisture content between 6 and 9 percent also increases price by 0.5% per percent moisture content. The basis for the ratio of impurities is 2%; every 1% difference means 1% change in the negative direction and in the positive direction too, up to 4%. The allowance for the difference in free fatty acid content is 1:1 price%:FFA%. Different standard is available for the sunflower seeds with high oleic acid content; the quality parameters and the limit values are the same that were for sunflower seeds with high linoleic acid content, but the oleic acid content should be higher than 85% in the fatty acid composition and the allowance is 1.5% price increase for every 1% increase up to 90%. The rapeseed varieties can be classified by their erucic acid content; the traditional varieties are rich in this fatty acid (about 50% in the total fatty acid content) and it also has significant glucosinolate content with a value between 90 and 140 µg/g. The limit value for glucosinolate content for human consumption is 5%. The varieties bred for low erucic acid content can be classified into four groups; the one with less than 5% erucic acid content (marked with “0”) has 4 to 5% less oil content than the traditional varieties (about 41 to 43%). The varieties marked with “00” have less than 2% erucic acid content and its glucosinolate content is decreased to 20-30 µg/g. erucic acid content of the varieties marked with “000” is less than 0.5% and the glucosinolate content is below 20 µg/g, while the “0000” varieties contains only traces of erucic acid and their glucosinolate content is less than 10 µg/g. With the decrease of these harmful components the oil content increases in the chemical composition, the “0000” varieties have their oil content higher than 45% (Table 10.). The erucic acid free varieties are rich in oleic acid, linolenic acid, tocopherols and sterols and have the lowest saturated fatty acid content. 9.2. táblázat - Table 10.: Chemical composition of different rapeseed variety groups (Lukács P, 1987 in Győri, 1995) Type Erucic acid in Oil content of Protein oil content, % seed, % in dm content of extracted meal, % dm Fibre and Glucosinolate shell in content of seed, extracted μg/g dm meal, % dm Traditional 48-52 45-47 37-38 17-19 90-140 Varieties marked with „0” under 5% 41-43 38-40 17-19 94-120 Varieties marked with „00” under 2% 42-44 38-40 12-14 20-30 Varieties marked with „000” under 0,5% 42-44 above 40 8-12 under 20 above 45 42-45 under 7 under 10 Varieties marked with „0000” in traces 63 Created by XMLmind XSL-FO Converter. 9. Requirements for industrial crops I. (sugar beet and oil plants) The Codex Alimentarius specifies rapeseed groups based on the fatty acid composition; low-erucic acid rapeseed oil must not contain more than 2% erucic acid (as % of total fatty acids); high oleic acid safflower oil must contain not less than 70% oleic acid (as a % of total fatty acids) and high oleic acid sunflower oil must contain not less than 75% oleic acid (as % of total fatty acids). The limit value for moisture content is the same 9% as for sunflower seed, but the limit value for oil content is less; 40% is the base value in the case of rapeseed. The quality requirements of soybean used for industrial purpose are declared in MSZ 6380-82 Hungarian Standard. It contains only requirement on moisture content from the chemical parameters; it should not exceed 14%. The other requirements deals with the purity of lot; 2% impurities are allowed at most (in which maximum 0.5% harmful can be present), and the kernels with reduced value are the shriveled kernels (with a maximum 5% ratio), sprouted kernels (with a maximum 2% ratio) and broken kernels (with a maximum 10% ratio). The USDA (United States Department of Agriculture) prescribe limits for heat damaged kernels and soybean kernels with different colour. The U.S. grading also do not have limit values for protein and oil content, but the general expectation is 35% protein content (ranging from 25 to 50%) and 19% oil content (ranging from 13 to 25%). 64 Created by XMLmind XSL-FO Converter. 10. fejezet - 10. Requirements for industrial crops II. (potato and tobacco) 1. Quality of potato The potato has been a staple food for a long time. Its carbohydrate content gives one of the bases of energy content of nutrition and its good storability makes the consumption possible in the whole year. Its importance shows connection to the economical development of a country; the consumption in developed countries significantly decreased in the latest 50 years but significantly increased in the developing ones. The reason for the widespread popularity of potato is that it can be consumed and processed in many ways. Varieties are available for cooking, frying and salad use for human consumption, the food industry uses it in the production of canned, cooled, frozen and dried potato products as well as in the production of starch and starch derivatives, due to the starch can be produced from potato most easily. It is also used for alcohol production and the tubers not suitable for human consumption can be used for animal feeding. The physical quality parameters of potato are the shape, size, colour, soundness and damage of tuber, the specific gravity of potato, the number and depth of buds, presence of rot, the uniformity and homogeneity of lot. The shape of potato can be roundish (when the ratio of width and height is nearly 1), oval (when the ratio of width and height is between 1 and 2) or long (when the ratio of width and height is higher than 2), depending on the variety and agronomy factors. The size of tubers is one of the basics of classification. The colour of flesh and skin can be evaluated; the flesh is yellow or white, while the skin shows much higher variability from write to pinkish and purple. The colour of skin and flesh is not in strong correlation to the potential use, but it is the base of preconceptions. The number and depth of buds is important for industrial use as they determine the ratio of peeling loss. The germinating eye-bud is a value-reducing factor. The soundness of tubers is important due to the resistance to spoilage during storage, although the suberification protect against the degradation to a certain degree. The tuber is exposed to microbial degradation due to the high moisture content and their occurrences also have physical signs. The uniformity of the tubers in the lot is important both for industrial use and fresh consumption and the lot should contain the same variety only. The density or specific gravity of tubers refer to their chemical composition and can be used for the estimation of moisture content and starch content. The chemical composition of potato can be characterized by the high water content; it ranges from 70 to 82% (24% in general), but the natural postharvest processes of tuber make it possible for a long-term storage in favourable conditions despite the high moisture content. The carbohydrate content is the highest one in the dry matter content and the starch is the one of it with the highest concentration. The starch content of potato tuber is between 12 and 20% in general, but 5 to 7% starch content for primeur potatoes and values higher than 20% for industrial potatoes are also published. The starch content what is lower than the value typical of the varieties makes the storability worse. The amylose-amylopectin ratio is about 1:3, similar to those of cereals. The sugar content is about 1% and storage may conditions significantly influences its value. The fiber content is nearly 1% also, the concentration of dietary fibers is higher than 2% on fresh matter base. The protein content of potato is about 2%, but the relative low concentration is compensated with its high biological value. The protein with the highest concentration is the tuberin, a globulin-like one, has high essential amino acid content and complements the cereal originated proteins in human consumption. More than 90% if it is utilized in the human body. The lipid content of potato is negligible, but its ash content is about 1% with significant potassium content, but the concentrations of phosphorus and magnesium are also high. The vitamin C, B1 and B6 and folic acid contents of potato are also important. The solanine, a highly toxic substance typical of Solanaceae, is present in the potato plant with high concentration in the fruit and green plant parts, but fortunately the tubers contain only a small amount in the peel, therefore it can be removed by preprocessing. On the other hand, solanine is sensitive to heat, thus the cooking or frying rapidly inactivates it. The green tuber flesh may refer to higher solanine content. 65 Created by XMLmind XSL-FO Converter. 10. Requirements for industrial crops II. (potato and tobacco) Summarizing the nutritional value of potato its importance is because the high and easily digestible starch and protein contents, favourable amino acid composition, significant dietary fiber and vitamin content and low fat content. 2. Classification and qualification of potato The basis for classification of potatoes is the use. The lots for fresh home consumption can be grouped by length of vegetation period; early, medium and late varieties are available; the food uses differentiate primeur or new potato what can be used for making salads and boiling. The skin and flesh colour has no importance in the use, but in several cases the ones with red skin and white flesh are more popular for cooking and the ones with yellowish skin and flesh are rather used for frying. Based on recommendations, the yellow potatoes are typically „all purpose”: ones, suitable for mashing, steaming, cooking, baking, roasting and frying, the red potatoes are good for steaming, cooking, roasting, making of au gratin, scalloped potato and salads. The russet potatoes can be used for baking, and cooking especially, while the white potatoes are suitable for mashing, cooking, steaming, roasting and au gratin making. The blue potatoes can be applied for steaming, baking and cooking. Practical classification is based on the cooking type (or the suitability for cooking) what takes into consideration the chemical properties for the determination of possible use. Three groups can be separated; • A: suitable for salad making. The texture has fine structure, flouriness cannot be experienced, has high moisture content or feels moist. The starch content is low or moderate, the water and in some cases the sugar content is high usually. These potatoes are also called waxy potatoes. Potatoes from this variety group fit for salads, cold dishes, baked potato casserole preparation. • B: suitable for cooking without disintegration („all purpose”): The texture is slightly floury and small granules can be felt. The texture remains stable during cooking and the potato does not disintegrate after cooking under moderate stress. Tubers of these varieties are suitable for cooking, salads and ragouts preparation • C: suitable for frying, disintegrating slightly during cooking: the flesh is coarse, feels dry. The tuber disintegrates after cooking or slight stress results disintegration. The starch content is high, therefore they are also called starchy potatoes. Their water content is relatively low. These kinds are suitable for frying, potato noodles, potato chips and French fries preparation and raw material for puree or flake production. The fourth group is this classification (D) is for industrial use but also available for other consumers. These varieties are the raw materials for starch, puree, chips and flake production. The primeur potatoes are harvested in the beginning of the season before full maturity. They have lower starch contents and higher water and sugar content than the ones harvested in full maturity and their skin can be removed without peeling. For the trade of potatoes minimal requirements and classification parameters are had to fulfil. The general requirements on every lot traded for food use are declared by the UNECE standard FFV-52 concerning the marketing and commercial quality control of early and ware potatoes. In Europe the R.U.C.I.P. (Rules & Practices of the Inter-European Trade in Potatoes), a French-based organization regulates the potato trade and, for example, the Hungarian National Potato Product Board also applies this reference for basis of trade. The R.U.C.I.P. defines requirements five categories; for seed potatoes, new potatoes, ware potatoes, industrial potatoes for processing into products for human consumption and industrial potatoes destined for the production of alcohol or animal feed. The general requirements are that the tubers must be normal appearance for the variety in shape, size, skin and flesh colour. The tubers should be intact, sound, healthy, practically clean and sufficiently firm. They have to be free of abnormal external moisture and adequately “dried” if they have been washed previously. They also have to be free of any foreign smell and/or taste. They have to be free of external or internal defects, such as brown stains, cracks, cuts, bites, bruise or roughness exceeding 4 mm in depth, They have to be free of green coloration, serious deformities, sub-epidermal and surface stains, hollow or black hearts and other internal defects. They have to be free of common and powdery potato scab up to 2 mm depth and it can be found on a quarter of the surface at most and frost damage, freezing injuries and heat damages cannot be observable. A partial absence of the skin is possible for the early potatoes, but the skin has to be fully developed. The surface has to be intact after suberification. Sprouting is not allowed. The tubers have to be developed sufficiently such as they withstand the transportation and handling and arrive. The lots have to be free from waste and foreign 66 Created by XMLmind XSL-FO Converter. 10. Requirements for industrial crops II. (potato and tobacco) matters. The packages, containers have to be labelled containing the information required for identification such as name and address of packer and/or dispatcher, nature (type, variety, commercial name) and origin of produce, commercial information (size, weight, optional properties) and optionally mark of official control. The shape of tubers should be typical for the variety. The common defects of shape are the growth cracks and other deformations (dumbbell, curved, pointed and knobby forms). The most common reasons for these effects are the suboptimal environmental conditions; the coarse texture of soil, the inefficient fertilization (not suitable ratio of nitrogen and potassium, but the lack of boron also increase the frequency of occurrence of these symptoms), virus infections, sudden changes of periods with high and low temperature, but the water supply is the most frequent cause for shape defects. The non-uniform soil moisture content, especially when moist conditions suddenly follows drought, easily leads to growth cracks therefore the soil water management is the key for preventing shape defects. The classification of potatoes can be based on size. The classification of OECD declares minimum sizes for fresh potatoes: the early potatoes have to be higher than 28x28 mm, the ware potatoes have to be higher than 35x35 mm and the size of long varieties have to exceed 30x30 mm. The maximum size for early and ware potatoes is 80x80 mm and 75x75 mm for long ones. The different kinds of industrial use defines their own requirements; for the production of French fries the size of tubers must exceed 55 mm, the size of the ones used for chips making must be between 41 and 55 mm and for puree making the tuber size must be less than 40 mm. 3. Industrial qualification Informative and easily determinable quality parameter is the moisture content of tubers. It generally ranges from 76 to 82%, the early varieties and earlier harvested lots have higher moisture content and during storage the value of this parameter decreases. The moisture content is in correlation with the density of tubers what can be determined rapidly by the determination of weight measured in water. For the analysis a specially calibrated measuring rod with a hook on the end is required. Specific mass (e.g. 3.6 kg) of potato has to be measured into a basket, it has to be hanged on the hook and immersed into room temperature water. Both the specific gravity and the weight measured in water can be directly read from the immersion depth of measuring rod. The 18 to 25% dry matter content is equivalent to 325 to 450 g weight measured in water. The requirements on moisture content of specific uses are 20 to 24% for French fries, 22 to 24% for chips, potato powder and puree making and low (less than 21%) for canning to avoid from boiling destruction. The associated values for weights measured in water are 370 to 450 g for French fries, 400 to 470 g for chips and 400 to 450 g for puree and flakes. The specific gravity is also used in practice and it can be calculated as The advantage of the use of specific gravity instead of weight measured in water is that it is independent from the way of determination (weighing). Another quality reducing parameter is the occurrence of flesh browning. It can be physiological or technological originated. The physiological browning has enzymatic causes. mechanical effects or shocks result cell damage and mixing of different cellular contents. Most often the phenolic compounds (e.g. tyrosine) are enzymatically oxidized (e.g. by polyphenol oxidase) then it ulinked to amino acids and proteins forming melanin. The exposure of tubers to enzymatic browning depends on external factors, for example fertilization and temperature at harvest by their effects on dry matter content and elasticity of tissues. The non-enzymatic browning (also known as cooking or frying browning) occurs as the effect of heating. Chlorogenic acid releases over 80 ° C and reacts with Fe ions and it results gray-black discoloration of potato flesh. The main factors of this deterioration are the fertilization and storage conditions by their effects on the citric acid content and chlorogenic acid - iron ratio. The reducing sugar content of tubers also has to be sufficiently low. The reducing sugars react with free amino acids during frying (Maillard reaction) resulting brown colouring and bitter taste of product. As free amino acids present in the fresh and stored tubers also, the concentration of sugar component will determine the intensity of reaction. The limit value for reducing sugar content is 0.2% for chips production and 0.3% for raw materials of French fries and flakes. This value may be influenced by the moisture content as the reaction shows the highest intensity at the end of frying operation, therefore the higher water content protects the potato against the browning. The maturity at harvest and the storage conditions (especially the temperature) influence it mostly. 67 Created by XMLmind XSL-FO Converter. 10. Requirements for industrial crops II. (potato and tobacco) The reducing sugar content can be determined by time-consuming titrimetric method, but test stripes are also developed for the rapid and chemical-free determination giving result within two minutes showing colour change. The suitability for frying can be considered at most by frying test. The potato is washed and peeled then it is cut and fried in rapeseed oil at 180°C for 3 minutes. The oil is soaked away from the surface of fried potatoes and they are compared to a colour table of sample fried products. The food safety related regulations give limit values for residues of sprout inhibitors, nitrate and glycoalkaloids. The most important glycoalkaloid is the solanine. It plays role in the natural defense of plant against fungi and pests, but it is also toxic for humans. The most parts of plant contain it, but the highest concentration scan be found the fruit and green parts of plant and only a small amount of it is in the skin of tuber. The green tubers contain more solanine; the tubers become green when they are exposed to light, therefore the proper storage hinders the increase of its concentration. Frying as heat treatment significantly lower its value, but the boiling has no effect on it. The limit value for total glycoalkaloid content is 100 mg/kg for the fresh potatoes. 4. Quality of tobacco Tobacco is the common name for the plants of Nicotiana genus and the word is also used for the products made from the leaves of these plants. It is used as a stimulant (cigarette, cigar, pipe and other ways) but it is also used industrially as pesticide and raw material for medicines. Several species are described but the use of main types is common in practice: • The Virginia or bright type has soft, mild and light flavor. The name “bright” comes from its colour what is light golden yellow or dark orange. Its popularity is due to its ability for artificial drying and rapid fermentation time beside the pleasant taste • The colour of Burley or brown type varies from light to dark brown. It has rather strong taste comparing to the other main types therefore it gives character to the product. It can be dried only naturally, therefore it and the curing lasts for a relatively long time. In contrast to the other groups the leaves of Burley tobacco is harvested with the stem as whole plant. • The oriental type tobaccos are the most aromatic ones with lower nicotine content. The small leaves have finer texture and harvest separately and processed by natural drying, but while the Burley is dried in chambers the oriental type is dried in sunshine. The EC Commission Regulation No 660/2006 (of 27 April 2006) specifies eight trade categories and lists the varieties are classified in the groups: I. Flue-cured (e.g. Virginia, Bright, Wika) II. Light air-cured (e.g. Burley, Bachus, Maryland) III. Dark air-cured (e.g. Havanna, Paraguay, Goyano) IV. Fire-cured (e.g. Kentucky, Salento) V. Sun-cured (Oriental type) (e.g. Xanthi-Yaka, Samsun) VI. Basmas VII. Katerini and similar varieties VIII. Kaba koulak (CLASSIC) The chemical composition of tobacco can be presented for the green (fresh) and the processed leaves and the seedling height has also a huge influence on it. The green leaves have high moisture content, it is between 80 and 88% what decreases to 23 to 14% after the drying process. The low moisture content is important for maintaining the storability of tobacco. The most important components of dry matter content are the carbohydrates and nitrogen containing compounds. The green leaves contains significant amount of starch (about 30% on dry matter base for Virginia) but it decreases to the fifth-sixth part during drying and curing due to the catabolic respiration. The ratio of sugars increases during the processing from 6 to 10% up to its double or 68 Created by XMLmind XSL-FO Converter. 10. Requirements for industrial crops II. (potato and tobacco) threefold. The fiber content of leaves is about 6 to 8% and the processing does not influence its value. The higher cellulose content makes the smoke flavor spicy and the quality worse, but it improves the burning capability. The nitrogen content of green leaves is about 1% and the protein content is 0.5 to 0.7% and during drying and curing the ratio of organic nitrogen compounds decreases. The ratio of amino acids also increases within the nitrogen containing compounds but their composition changes, especially the one of proline which may increase to twentyfold. The ash content of fresh leaves is between 9 and 25% of the dry matter. The Ca and K contents increases mostly by fertilization, but the present chlorides and phosphates make the burning properties wrong. The organic acids have important role in the sensory properties but the main acids of different types are different (e.g. malic acid, citric acid, oxalic acid) and their changes during processing are different; the total acid content of artificially dried tobacco leaves is decrease while this value increased for the naturally fermented leaves. The aromatic violate compounds are also have importance in the consumer’s acceptance and the vegetable resins: improve burning properties. The tobacco also contains alkaloids; nicotine is the most important of them. Its concentration is between 0.5 and 8%, but the processing significantly decreases its concentration. The leaves from the different seedling heights have their special names and it has influence on their chemical composition, therefore their quality and value. The leaves on the lowest part of stem are the voledo leaves. They are the ones below the sixth leaf, have the lowest nicotine content (about 1%). The seco leaves are the ones from the 6th to the 15th leaf and the ligero leaves are the ones from the 16th leaf. The nicotine content increases upwards rapidly to the seco leaves but this increase slows down in the region of ligero leaves. The sugar content is the highest in the zone of seco leaves, it reaches 25%, bit decreases both upwards and downwards to about 5%. Ligero leaves have coarse structure and the burning properties of voledo leaves are the best but it is poor in aroma. The position of leaves made a tobacco product from can be a marketing tool referring to the quality. The most important external factors influencing and characterize the quality of tobacco are: • leaf size, shape: typical of the variety or the type, ovate or lanceolate • thickness: influenced by the growing conditions. Leaves from cohesive soils are coarser and the ones from light soils are finer. • vascularity: the higher thickness of the main vein makes quality worse. The side veins should not stand out from the surface. • structure: the hygroscopicity and burning ability are the most important structure related quality parameters. The drying significantly decreases the hygroscopicity of leaves and the dried leaf can be considered as nonhygroscopic. In contrast, the burning ability improves during the curing due to the chemical changes such as mineralization of organic components. • condition of leaf surface • flexibility, elasticity: mainly influenced by the pectin content and composition. During curing they degrade but the insoluble pectin forms are more stable, therefore the flexibility of leaves increase • colour and light: during the drying the chlorophyll decomposes and the yellow or brown colour appears. The Maillard reaction and the formation of melaniods compounds during curing result further changes in colour. Patchiness is undesired but can be corrected during curing. • integrity, health: leaves with incomplete surface or damaged by pests or diseases are defective ones. • maturity: the physiological and technological maturity are not the same. The tobacco leaf for processing can be considered as matured when the leaf start to fade, the main vein becomes bright, the leaf becomes fluted and waxy and it is easily breakable. The internal factors influencing end-use are the followings: moisture content, total nitrogen and organic nitrogen content, total sugars and reducing sugars (due their effects on the acidity or alkalinity of smoke), the amount of alkaloids, nitrate and chloride content, combustibility (it can be improved by curing), aroma and flavor of the tobacco and its smoke, pH of smoke. The pH can be classified as acidic (e.g. oriental type, Virginia), alkaline (e.g. Burley) or neutral. 5. Qualification of tobacco 69 Created by XMLmind XSL-FO Converter. 10. Requirements for industrial crops II. (potato and tobacco) The MSZ 16802:2001 Hungarian standard lists the quality requirements and grades of cured leaves of tobacco. The general requirements are that the leaves in one lot have to be from the same variety. The leaves have to be free from mildew and the ones in the same quality class should not be mixed to others from an another quality class. The scion leaves and tendrils (grow from side shoots) and their parts should not be classified into quality groups The standard separates four main groups, declares seedling heights and gives limit values for damages, colour defects, maturity, leaf texture and texture quality, leaf shape and specific demands (e.g. insect or technological damages): 1. Artificially cured Virginia type varieties • subgroups by quality (AV, BV, B, I, ZV, B II., B. III. C) and their seeding heights 2. Naturally cured Burley type varieties • subgroups by quality (A, B, C, D) and their seeding heights 3. Naturally light air-cured type of light cigarette varieties • subgroups by quality (A, B, C, D) and their seeding heights 4. Naturally cured type cigar varieties • subgroups by quality (A, B, C, D) and their seeding heights The extension of disease and injuries is expressed by the ratio of the damaged (missing) or diseased leaf area and the whole surface of the leaf. In these categories, the incomplete leaves or leaf blade damaged by pests and diseases can be considered as well as the leaves are affected by thrips damage and the leaves suffering from frost and hail damage. 70 Created by XMLmind XSL-FO Converter. 11. fejezet - 11. Requirements for fruits and vegetables The product groups of fruits and vegetables contain those plants a part of which is edible freshly or after domestic processing (e.g. boiling). The edible part of vegetables can be their root (e.g. carrot, radish), stem (e.g. onion and kohlrabi), leaf (e.g. parsley, cabbage), flower (e.g. cauliflower), or fruit (e.g. tomato and cucumber), but in the case of fruits the consumed part of plant is the fruit. The vegetables are herbaceous plants but the group of fruits contains both herbaceous and woody ones. 1. Chemical composition of fruits and vegetables Table 11.and Table 12. present the chemical composition of main fruits and vegetables. The most important properties of them are the high moisture content considering the storability and possible spoilages. The moisture content is ranged from 80 to 97% generally, but some groups show difference due to a specific chemical component; for example the pulses and the garlic are rich in carbohydrates and proteins and the nuts are rich in lipids. The moisture content of nuts is less than 50% due to the high lipid content and it is often below 10%, e.g. for walnut, peanut and almonds. The high moisture content results that the dry matter contents of these products are low, therefore they are not the mains sources of energy and macronutrients but the high water content also has benefits; the digestibility and availability of nutrients are high. The high moisture content results that these products are sensitive to the air humidity during storage; the typical water activity for most fruits and vegetables are higher than 0.9 and 0.95. So high relative air humidity should be kept during their storage that would result high degree of microbiological spoilage and the climacteric ripening would not occurred as normal way, therefore the relative air humidity is lower than the equilibrium what result shrinkage losses. Carbohydrates are the chemical compounds what present in the highest degree in the dry matter content. The sugars and polysaccharides are also important components of fruits and vegetables. The sugars plays role in the flavor of fruits. Glucose, fructose and sucrose are the main sugars of fruits; but sugar alcohols such as sorbitol and xylitol have also high influence. The different fruits have different sugar and sugar alcohol ratios what modifies the taste experienced. The sugar content of fruits is between 5 and 20%; banana and grape have the highest values. The distribution of sugar is not homogeneous in the flesh as well as in the complex produce of grape; the brunch of grape has a specific sugar distribution due to the arrangement and distance of grape berries from the pedicle. The vegetables are typically poorer in sugars, but the fruits (e.g. paprika, tomato) and some other products (sweet corn, green pea) are also rich in them. The polysaccharides play role in the physical structure of products. The insoluble fibers (mainly cellulose, hemicellulose and lignin in fruits and vegetables) are resistant to the processes occur during storage but their microbiological degradation result undesired changes in structure, for example in the case of fermented vegetables. The pectin is the main soluble fiber of these products. In the earlier maturity stages its insoluble forms are dominant resulting flexibility for the tissues but during ripening there is a shift in the ratio toward to the water soluble forms resulting softening in tissues. The pectin is also exposed to enzymatic destruction (pectinesterase) resulting tissue firming. Starch is the main storage polysaccharide of fruits and vegetables, but it decomposed during ripening of fruits. Beside sugars organic acids also have important role in the flavor. The main organic acids of horticultural products are malic acid and citric acid for pome fruits, citric acid and isocitric acid for berries and succinic acid for stone fruits (e.g. plum). Oxalic acid also present, mainly in the green leaves of vegetables. The concentration of free organic acids is relative low in fresh products, more than 90% of them is in salt formed with mono and divalent ions (mainly Ca, Mg, K). The concentrations of organic acids are relatively stable until harvest but it continuously decreases during storage. For the proper sense of taste the ratio of sugars and organic acids is important; then it is 10:1 it is ideal for apple, 6:1 is required for sour cherry. The concentrations of nitrogen containing compounds are generally low for these products (about 1% in the fresh material), but the pulses from vegetables and nuts from fruits have higher protein contents. The ratios of inorganic and organic nitrogen compounds are varied; about 40 to 90% are organic form. The nitrite and nitrate are the potentially dangerous inorganic forms of vegetables but it is also important to remember that every 71 Created by XMLmind XSL-FO Converter. 11. Requirements for fruits and vegetables vegetable product has its typical nitrate content. For example radishes, lettuce and spinach are typical nitrate accumulator plants and generally the green plant parts and storage organs contain higher levels of nitrate while flowers and fruits are poor in it. It is strongly influenced by the conditions of production; when high nitrogen fertilizer doses are applied and there are not enough sunlight providing energy to transform the inorganic nitrogen forms to organic ones, wet and cool is the weather the accumulation of nitrate is much more frequent. The vitamin content of fruits and vegetables highly increase their biological value. They contain almost all the vitamins except B12 and D, but the concentrations of vitamin C and β carotene are outstanding. The mineral element contents of fruits and vegetables are low due to the high moisture content but they are rich in potassium and low in sodium improving the physiologically important Na/K ratio what is shifted to the sodium due to the inappropriate eating habits. It is also important to mention their magnesium and calcium content, but they are rich sources of trace elements, especially iron, zinc and copper and the absorption of them is easy therefore their utilization ratios are high in the human body. The different organic antioxidants are present in high concentrations in some of the horticultural products. Summarizing the nutritional benefits of fruits and vegetables they have important role in the fiber, vitamin and antioxidant intake while their consumption contributes the mineral element balance of people. It is also important that there are poor in harmful or potentially harmful substances. The most frequents are the cyanoglycosides, e.g. the amygdalin occurs in the stones of hard-shell and pome fruits, the prunazin occurs in small amounts in flesh of hard-shell fruits or the sambunigrin occurs in the flesh of elderberry. Prunetin what is an isoflavonoid having female hormone action was detected in hard-shell fruits also. The lectin has harmful effect on red blood cells occurs in quince and sweet cherry Oxalic acid may results to kidney stone or kidney sand. In some cases the fruits cause allergenic reactions; the histamine content of peaches, apricots and strawberries may result symptoms for children mainly. The pollens of apple are also allergenic for sensitive persons. The tropical fruits (banana, citrus, kiwi, Mandan, papaya and lychee) have most often allergenic effects. The sulfur content of some vegetables (alliums, horseradish and mustard) may also causes symptoms. There are several complex indexes to valuate and ranking the different fruit and vegetable species based on their health benefits. The Average Nutritional Value developed for vegetables is one of them. It considers the fiber and protein contents from macronutrients, calcium and iron from the mineral elements and vitamin C and carotene content from vitamins, indicating that the nutritional benefits of these plant products are based on these components mainly. The sweet pepper, lettuce and carrots also show high values while the cucumber, watermelon and tomato have low or moderated values. 11.1. táblázat - Table 11.: Chemical composition of main vegetables (Győri, 1999) Moisture content, Protein % % content, Lipid content, % Carbohydrate content, % Ash content, % carrots 88,1 - 91,9 0,7-1,2 0,10-0,30 5,8-8,2 0,66-1,0 horseradish - 2,0 -3,7 0,30 -0,31 - - radish 93,3 - 95,5 0,84 - 1,2 0,10 -0,20 1,9 - 4,0 0,79 - 1,0 red beet 82,9 - 91,7 1,1 - 2,0 0,10- 0,20 6,8 - 8,7 0,77 - 1,1 celery 87,3 - 90,5 1,2 -2,0 0,20 -0,46 4,8 - 9,0 0,91 - 0,97 cabbage 91,0 - 93,0 1,2 - 1,5 0,10 -0,20 3,5 - 4,3 0,37 - 0,8 red cabbage 91,5 - 92,4 1,4 - 1,7 0,10 -0,20 3,9 - 5,0 0,50 - 0,80 cole 88,0 - 91,8 2,6 - 3,4 0,27 - 0,60 3,5 - 5,0 1,0 - 1,2 cauliflower 90,9 - 93,0 2,0 -2,7 0,20 - 0,31 3,0 - 4,6 0,80 - 0,83 72 Created by XMLmind XSL-FO Converter. 11. Requirements for fruits and vegetables broccoli 88,7 -90,4 - - 4,2 - 5,0 - chinese cabbage - 1,17 - 1,20 - - 0,59 - 0,70 brussels sprouts 83,7 - 86,3 3,9 - 5,0 0,42 - 0,69 6,0 -7,6 1,3 - 1,5 kohlrabi 90,0 - 92,0 1,6 - 2,3 - 3,9 - 5,6 0,89 - 1,0 mangold 91,0 - 94,0 1,4 -2,6 0,10 - 0,42 2,8 - 4,0 0,20 - 2,2 lettuce 94,0 - 95,9 1,2- 2,3 0,20 -0,31 0,1 - 2,3 0,43 - 1,40 spinach 88,9 - 93,3 2,0 - 3,0 0,20 - 0,41 0,5 - 2,7 1,40 - 1,90 asparagus 93,0 - 94,0 1,5 - 2,2 0,10 -0,20 2,0 - 3,2 0,54 - 0,80 green beans 88,9 - 91,5 2,0 - 3,0 0,20 -0,40 2,9 - 6,4 0,68 - 0,-80 green peas 74,3 - 77,7 6,0 - 7,2 0,40 -0,50 12,0- 15,5 0,90 - 1,10 cucumber 96,1 - 97,3 0,5 - 0,8 0,05 -0,30 1,0 - 2,2 0,40 - 0,89 pumpkin 90,3 - 93,0 1,0 -1,2 0,10 - 0,20 - 0,73 - 0,80 sweet corn 73,9 - 75,6 2,9 - 3,7 - 18,6 - 19,7 0,70 - 0,93 green peppers 87,0 - 93,0 0,7 -1,5 0,20 - 0,60 3,3 -8,0 0,50 -0,70 tomato 93,4 - 95,2 0,69 - 1,0 0,20 - 0,30 1,9 - 4,0 0,60 -0,61 onion 86,0 - 89,0 1,0 - 1,4 0,10 - 0,40 9,4 - 10,0 0,57 - 0,60 garlic 63,0 - 64,6 5,3 - 6,8 0,06 - 0,20 22,6 - 27,9 1,40 -1,44 leek 86,3 - 90,8 2,0 - 2,5 0,25 - 0,44 4,6 - 9,2 0,82 - 0,90 11.2. táblázat - Table 12.: Chemical composition of main fruits (Győri, 1999) Protein % content, Acid content,% Carbohydrate content, % Moisture content, Ash content,% % apple 0,4 0,4 7 90,5 0,4 pear 0,4 0,3 12,0 83,8 0,4 quince 0,6 0,9 12,0 83,9 0,6 medlar 0,6 1,1 12,0 82,7 0,8 apricots 0,9 1,3 10,2 86,0 0,7 peach 0,7 0,8 9,0 87,9 0,6 73 Created by XMLmind XSL-FO Converter. 11. Requirements for fruits and vegetables cherry 0,8 0,7 14,0 83,6 0,5 sour cherry 0,8 1,6 11,0 85,7 0,6 plum 0,7 0,5 13,1 84,7 0,5 grape 0,6 0,5 18,0 79,1 0,5 gooseberry 0,6 1,4 8,0 89,4 0,6 redcurrants 0,6 2,5 7,0 89,2 0,7 blackcurrant 0,9 2,8 9,3 86,2 0,6 strawberries 0,9 1,4 7,2 89,0 0,7 raspberry 1,2 1,2 5,4 86, 0,6 melon 0,5 0,1 9,5 88,4 0,8 watermelon 0,5 0,2 6,5 91,5 0,5 orange 0,9 1,5 7,0 89,6 0,5 mandarine 0,8 0,8 8,0 91,1 0,6 lemon 0,7 5,5 2,3 88,7 0,6 figs 3,3 1,3 59,8 26,1 2,5 banana 1,3 0,1 22,8 74,1 0,9 Protein Lipid Carbohydrate Moisture Ash walnut 18,6 57,0 11,7 8,1 1,7 peanut 13,6 63,5 8,7 2,0 2,0 almonds 27,6 52,2 6,8 6,5 3,2 2. Requirements on fruits and vegetables The standards regulate the quality requirements of fruits and vegetables are treat commonly these plant originated products in several cases due to the similarities of their chemical composition, physical properties and requirements on external conditions. The EC No 1234/2007 (22 October 2007) establishing a common organisation of agricultural markets and on specific provisions for certain agricultural products regulation with the Commission implementing regulation (EU) 543/2011 (7 June 2011) contain the general quality requirements on the fruits and vegetables traded in the European Union. It has horizontal regulations (general minimum requirements on quality, ripening, tolerance and marking of origin) and product specific regulations for apple, citrus fruit, kiwifruit, lettuces, curled leaved and broad-leaved endives, peaches and nectarines, pears, strawberries, sweet peppers, table grapes and tomatoes. The regulation says that fruits and vegetables can be traded only when they are intact, healthy, commercial quality and the country of origin is marked. It declares such general requirements what can be applied for those products what are not regulated separately. 74 Created by XMLmind XSL-FO Converter. 11. Requirements for fruits and vegetables The Codex Alimentarius also word requirements on the quality of fruits and vegetables, partly overlapping with the EC regulations (for example table grape or apple) but partly for other fruits (e.g. gooseberry, banana and litchi, but the full list can be found at http://www.codexalimentarius.org/standards/list-of-standards/en). For those products about what there is no EC regulation or Codex standard available, the standards and recommendations of United Nations Economic Commission for Europe (EN UNECE) can be applied. UNECE standards are available for several fruits and vegetables but a so-called blank standard layout is offered what can be filled with the contracting parties. The UNECE standards and recommendations are available at http://www.unece.org/trade/agr/standard/fresh/ffv-standardse.html. Currently 50 standards are available on the UNECE website defining recommendations for the most common fruits and vegetables, for example apple, citrus, kiwi, peach, nectarine, pear, strawberries, table grapes, and for sweet peppers, lettuces, curled leaved and broad-leaved endives, tomatoes, and others. The OECD prepares detailed brochures based on the UNECE standards and richly illustrates the different colouring, damage types and other requirements. These brochures can be found at http://www.oecd.org/tad/standardsforseedstractorsforestfruitandvegetables/oecdfruitandvegetablesstandardsbroc hures.html. The structure and requirements of different sources of standards and regulations (UNECE, Codex Alimentarius and European Commission) are similar. All of them declare that fresh fruits and vegetables van be purchased solely if they are unharmed and healthy ones, commercial grade and the country of origin is indicated. All the products have to fulfill the general and product specific regulations. Those products what do not fit the specifications cannot be marketed. For example the EEC No 920/89 (10 April 1989), Codex Alimentarius Hungaricus regulation 1-4-920/89/3 and the UNECE standard FFV-56 contains the same requirements for apple (and the Codex Alimentarius Hungaricus uses the EEC No 920/89 as reference). The structure is the following: • General requirements: they have to be valid for all the classes and grades. They provide that the products have to be intact, clean, free from foreign matters. They should be healthy and cannot be unfit for consumption or processing because of deterioration. They should be free from pests, damage caused by pests, free from abnormal external moisture and any foreign smell and/or taste. These satisfactory conditions have to be kept during the whole transportation and handling. • Requirements for maturity: the development of fruits has to be sufficient, satisfactory and the level of maturity must be such as to enable continue ripening. Morphological, physical, chemical, biological and environmental parameters can be evaluated for the determination of maturity • Classification: declaring the aspect for classification into Extra, Class I and Class II groups are separated (EEC No 920/89 also defines Class III). For classification, sizing (limit values for the lowest diameter of fruit), colouring, sound and tolerance values are defined for each quality class. Allowed rate of different kinds of defects (development, colouring, skin defects and russeting) and presentation are also defined. • Requirements for marking: the following information must be shown: • the country of origin, • the variety (e.g. for apple, but for other products trade group (e.g. for tomato) or flesh colour (e.g. peaches and nectarine) are required to mark). • Name and address of packer or distributor • Quality class The apple classified into the extra class has to be shown superior overall quality and all parameters (colour, size, shape) must be characteristic of the variety. The pedicle must be intact, the flesh must be absolutely healthy and only very slight skin defects and russetings are allowed. The regulation on apple defines 4 groups by colour. The group A varieties with red skin (e.g. Red delicious) and for the extra class three-fourth part of surface has to be coloured. The group B lists varieties with mixed red skin (e.g. Topaz, Jonathan, Idared, Florina) and the classifying into extra class half of the surface has to be covered by mixed red colour (typical of variety). The group C is the group of slightly coloured striped varieties (e.g. Elstar, Gála, Jonagold, Pinova) and the limit for surface colouring is one-third part for the extra class. The group D lists the other (non red) varieties (e.g. Golden Delicious, Granny Smith). 75 Created by XMLmind XSL-FO Converter. 11. Requirements for fruits and vegetables The size of apple is specified by diameter (measured at right angles to the axis of apple) or mass. The UNECE FFV 56 defines 60 mm or 90 g limit values for extra category but the fruits with lower size can be accepted if their Brix values are 10.5 °Brix at least and its size is not lower than 50 mm or 70 g. The EEC No 920/89 separates varieties with large fruits and others; the limit value for extra class is 70 mm for large fruits and 60 mm for other varieties and these limit values decrease by 5 mm for I., II. and III. classes. The practical requirements on peach for fresh consumption are that the fruits in the lot should be in uniform maturity stage. The flesh must have elastic texture and it should not be prone to browning nor in the flesh and by the stone. Fruits have to be easy to peel and flesh and stone should be easily separated. It is also important for industrial use, however, the most variety are not freestone. Generally other varieties are suitable for fresh consumption and industrial use; the flesh color, firm texture and freestone are the main industrial requirements on peach. In the case of apple the industrially important parameters are that the fruit has to be juicy with high acid content, not mushy, firm flesh, typical flavor, 55 mm diameter at least. The autumn and winter varieties are typically more favorable. In the case of pear firm flesh and high acidity are the requirements and the flesh should be not gritty. The flesh of sweet cherry should be hard and firm with dark red color. 15 mm diameter is the minimum requirement for sour cherry and it has to have painting colour. The raspberry has to be transported without peduncle, it must be rolling and not juicy. The requirements on pea are the colour (the peas should be dark green), free from injuries and impurities. Size categories are developed (6 to 8; 8 to 9; 9 to 10 and higher than 10 mm) and the most important qualification parameter is the tenderness of the peas determined by finometer or tenderometer. The categories are the following: 1. For freezing, tenderness measured by tenderometer • A below 110 T° • AB from 111 to 125 T° • B from 126 to 140 T° • C from 141 to 160 T° 2. For canning, tenderness measured by finometer • o from 37 to 45 F° • from 45 to 54 F° • from 55 to 64 F° • classless above 64 F° In the case of the tomatoes the regulations and requirements of UNECE and Europan Union are sounds very similar again. Both recommendations classify the varieties into 4 trade types by the form of fruits: round, ribbed, oblong or elongated and cherry tomatoes (including “cocktail” tomatoes). The general requirements are similar to those of the ones for apple; the general appearance of lots (purity, health and foreign matters) is defined. The classification declares extra, class I and class II groups. The fruits in the lots qualified into the extra class have to show superior quality, they have to be firm ones and fulfil to the characteristics of trade type or variety by shape, appearance, development and colouring. Fruits have to be free from greenbacks and other defects, except the insignificant ones which do not influence the general appearance, quality and storability of product. Sizing and sizing scale are declared as well as the tolerance values. The industrial requirements on cucumber for canning are that the raw materials have to be fresh-picked, their lengths have to be ranged from 3 to 12 cm long, the ovary has to be small, and thy have to be free from bittermaterials. Special, small, medium, long and salat categories are separated by the length and thickness of cucumber. 3. Ripening of fruits There are regulations on maturity stage, but the economic considerations and quality oriented production also require be able to judge the maturity stage of fruits. The fruits can be classified into tow groups by their 76 Created by XMLmind XSL-FO Converter. 11. Requirements for fruits and vegetables ripening; non climacteric and non-climacteric ones. The respiration rate of non-climacteric continuously decreases during ripening and after harvest this rate increase; therefore to protract the freshness only the late harvest can be used. The climacteric fruits also show decrease in their respiration rate, but after reaching a minimum value it starts to increase rapidly, reaches a maximum point (climactericum) then start to decrease even faster (Figure 15.). Mainly the size and mass of climacteric fruits increase before the minimum point, but afterwards the mass and volume increase are minimal in the climacteric respiration period, but most of the flavor and aromatic compounds synthetized and the texture, colour and chemical composition of flesh change favorable in this ripening stage. If the fruit is harvested before the increase of respiration rate the after-ripening will take place but the storability of fruits will be much longer. Examples for climacteric fruits are the apple, pear, peaches, nectarine, plum, mango and banana, while the berries, grape, citrus fruits, cherry and pineapple are non-climacteric fruits. The maturity stage is important not only for trade for fresh consumption but for the processing industry. In general it can be said that fruits before the 60% of full maturity are unfit for harvest, from 60 to 70% they fit for long transport or long-lasting storage, from 70 to 80% they fit for temporary storage and production of boiled fruits, from 80 to 90% they are suitable for freezing or fresh consumption, from 90 to 100% (fully ripening) they fit for prompt fresh consumption and juice and jam production. Over the full ripening stage their quality become worse, rapid spoilage endangers and they can be used for fermentation. 11.1. ábra - Figure 15.: Change of respiration rate of climacteric and non-climacteric fruits There are several methods for the estimation or determination of maturity stage. For estimation, the number of days after anthesis or the temperature sum can be bases, but other environmental factors highly influence its accuracy. Similarly, the mass or volume of fruits is also can be used for determination of maturity stage as well as it is a way of quality analysis. The comparison of fruit skin (the intensity, coverage rate of top colour) to samples is similarly applicable for the evaluation. Much more proper methods are the physical and chemical tests. The starch-sugar conversation can be evaluated rapidly and easily. The dry matter or sugar content of fruits measured by refractometer can be performed on field as well as the analysis of starch; for example when the apple cuts in half perpendicularly to the apple core and draws into iodine solution the starch in the flesh is painted to dark blue by the iodine and by the comparison of apple slice to a table presenting typical colourings for different maturity stages the preening stage can be objectively determined. The determination of total starch content can be performed by titrimetry and the sugar-acid ratio is also an objective parameter; for example 10 to 1 ratio is ideal for apple and 6 to 1 is for spur cherry. The firmness of product is also refers to the ripening stage, a penetrometer can be used in the orchards to objective analysis. The determination of juice content is used for the maturity analysis of citrus fruits. Modern physical measurements are under development for the evaluation of maturity stage. The spectrometry (UV, VIS and NIR) is used for different kinds of products but it is not widely used. Acoustic methods can also 77 Created by XMLmind XSL-FO Converter. 11. Requirements for fruits and vegetables be used; the flesh of fruits partially absorbs sound generated by punching it and the wavelength of transmitted sound refers to the chemical composition. Electrical measurements (based on resistance or conductivity) are also applied. 78 Created by XMLmind XSL-FO Converter. 12. fejezet - 12. Determination of meat quality I. (general considerations) The meat is sum of muscle tissues of animals, which is suitable for human consumption from the aspects of food science, that is the edible flesh of animals. Meat is meant as the muscle tissue of animals in the most common sense, but the processing and trading of meat include the adeps (originated from fat tissues) and intestines (heart, liver, lungs, kidney, tripe and stomach), tongue and blood. The meat can be originated from livestock and hunted animals. The main meat sources in human consumption are the domesticated animal species, of these the bovine, porcine, ovine, poultry and fish, but goats, horse, camel and other species cultivated in some areas with minor importance may also be significant. On the other hand, the game meat is also popular product having high consumer value but its role in processing is also minor. The quality of meat includes: • food safety issues: the character of meat, the chemical composition and the exposure to rapid spoilage requires strict hygienic regulations. Toxicological aspects are also have to be considered due to the elements of husbandry (drugs, parasites, pathogens, etc.) • nutritional value: the amount and composition of nutrients and other physiologically important components. • sensory properties: tenderness, flavor, colour, etc. • technological suitability: the conformity of a specific product to produce (pH, water holding capacity, ratio of muscle/fat tissue, etc.) The food safety and nutritional value related properties are mainly determined by the chemical and biological quality parameters of meat, while the sensory and technological properties are in relation to the physical parameters, but the chemical ones are also have influence. The tissues of animal products used in human consumption are the muscle tissues primarily, but the connective, fat tissues are present between the muscle tissues. The bones themselves are not edible parts of the animal bodies but they can also be used for food production (e.g. soup making). The nervous tissues are not consumed but some foods may contain them. The surface of muscle is covered by connective tissue membrane what percolates into the muscle. The muscle is composed of muscle fibers also surrounded by connective tissues and fat cells, resulting marbling in the meat. The muscle is built from muscle cells what having fiber shape and shows striated, therefore they are called striated muscle. The muscle fiber is a multi-nucleus individual unit of merged muscle cells. The part with the highest ratio of the muscle fiber is the myofibril, the contractile element of muscle. They are intercalated in the sarcoplasm, what contains glycogen, lipids, enzymes and proteins such as myoglobin. The sarcoplasmatic reticulum is formed by longitudinal and transverse tubes, contains calcium in terminal cisterns and surrounds the myofibrils. The myofibrils are from longitudinal sarcomers, in what a dark (anisotropic, A) zone is between two (isotropic, I) zones, and the latter one is pulled apart by a Z line. Both zone show filamentous structure; the dark zone has thick and thin filaments and the light zone has only thin filaments. The main component of thin filaments is a protein called actin and the one of the thick filament is the myosin (Figure 16.). The myosin shows adenosine triphosphate (ATP) activity and can connect to the actin forming an actomyosin complex. In the muscle in dormant state the actin and myosin do not connects and the muscle can be formed, it is extensile. When the muscle becomes active, the reacting heads of myosin connect to the actin, dissociate then connect again to next actin monomers (sliding filament theory). This results a closed actomyosin complex with shortened length of fiber, therefore a contracted muscle. In the relaxation stage the actomyosin complex decomposes due to the hydrolysis of ATP and the increase of the calcium concentration from the sarcoplasm reticulum. Both the active and relaxation stage requires ATP what is provided by its current presence and glycogen content of sarcoplasm. The knowledge on the structure of meat is important to understand the processes during and after slaughter. 12.1. ábra - Figure 16.: Structure of muscle 79 Created by XMLmind XSL-FO Converter. 12. Determination of meat quality I. (general considerations) The muscles in the body can be red or white muscles. The red muscles are suitable for the stable work, stressed more often with low efforts. They contain thinner fibers, more myoglobin and respiratory enzymes but less myofibrillum and carbohydrate. They have higher water content. They are able to do slow contraction and relaxation, capable to constant use and have low glycolitic but high respiratory activity. Red muscles are, for example, muscles of respiratory and digestive systems and posture. In contrast, the white muscles are able to rapid concentration and relaxation, periodic use and have high glycolitic activity, therefore capable for heavy but rare effort. Usually they have large masses, thicker fibers, more carbohydrate (glycogen) contents, less myoglobin and respiratory enzymes and lower water content. For example, the muscles of moving are white muscles. The different meats contain both red and white muscles in different ratio, what influences by the body part, age, sex, condition, husbandry and other factors. The connective tissues are mainly collagen and elastin. The collagen is rigid, non-elastic tissue. The collagen molecules are located parallel stepwise shifted and there are crossulinks amongst them. The stability of different collagen molecules (there are more than ten are known) are different, but the number and overall stability of crossulinks increase by age, therefore the structure of meat becomes firm and chewy, but it is strongly influenced by the species, husbandry and way of processing, as the heat resistances of crossulinks between collagen molecules are different. The number of collagen tissues is stable, does not increase by age, therefore this is not influence the meat quality. The elastin is an elastic connective tissue has long, branching fibers. It is more resistant to heat, mechanical and chemical effects than the collagen is. The fatty tissues are modified vesicular connective tissues. Fat droplets appear in the vesicular connective tissue, they increase and merge later. The fatty tissues are present in two main forms in the animal body; membrane and reserve fats. The membrane fats are built mainly from unsaturated fatty acids, but the composition of reserve fats is influenced by the fatty acid composition of forage. In the qualification and food use the reserve fats are the important ones. Its main forms are the fat under the skin called subcutaneous fat (adeps), fat around the intestines, the intermuscular fat (or light fat) and intramuscular fat (what result the marbling of meat). The marbling is an important sensory quality parameter of meat. The pinguescence of different species, varieties and meat kinds is different and the composition of forage significantly influences it. 1. Post mortem processes in meat and their effects on its quality The most important direct effect of slaughter on the quality of meat is the stop of blood circulation. The muscle function is required energy; the ATP synthesis can be done by respiration and glycolysis. In the lack of blood circulation there is no oxygen supply for the respiration, therefore it stops and glycolysis starts or its speed increases. The glycolysis produces lactic acid and the lack of blood circulation it cannot transferred. Its increasing concentration in muscle decreases its pH. The quality of meat is highly influenced by the amount of energy sources compounds (ATP, creatine phosphate) in the slaughter. The stop of respiration terminates the ATP synthesis, therefore the energy supply of muscle function starts to decrease from this source. In the lack of energy the actin and myosin connects as actomyosin 80 Created by XMLmind XSL-FO Converter. 12. Determination of meat quality I. (general considerations) permanently resulting rigor mortis. The muscle becomes rigid, non-extensible. The lack of ATP and pH decrease lead to the denaturation of proteins resulting water losses (water drop). The protein destruction is also a consequence of pH decrease and its visual effect of fading as the result of myoglobin destruction. Later the actomyosine complex also splits due to the proteolysis resulting softening. The stop of blood circulation and the water and metabolites release from these processes provide favorable circumstances for the germ number to increase (Figure 17.). 12.2. ábra - Figure 17.: Post mortem processes in meat (Dióspatonyi I., http://www.chemonet.hu) Due to the glycolysis the pH of meat decreases from the physiological 7.2 to 5.5 to 5.6. The speed of pH decrease is influenced by the post mortem processes; if there are available sufficiently amount of high-energy phosphate bonds then the speed of glycolysis increases slowly resulting relatively slow pH decrease. When low amount of macroerg phosphates is available after slaughtering the more rapid glycolysis results more intensive pH decrease, but when there is no glycogen available then the glycolysis does not take place, therefore the decrease of pH is minimal. The pH is measured two times for monitoring the post mortem processes; 45 minutes and 24 hours after slaughter. In the meats show normal quality the decrease of pH is almost continuous, more rapid in the beginning and slows down to the 24th hour. The meats with more rapid glycolysis show intensive pH decrease; its pH value is less than 6.0 by the 45 th minutes and less than 5.6 after 24 hours. The pH of meats without glycogen reserves measured in the 45th minute is a little bit less than 7, and it does not decrease below 6. The final pH is between 5.5 and 5.6 and the time necessary to reaching it depends on the species; the pH decrease is slower in cattle meat and faster in porcine meat. The most important physical quality parameters of meats are the water holding capacity, the colour and the texture. The water holding capacity of meat is influenced by the speed of pH decrease; when it is rapid the ratio of denaturation of proteins is higher resulting rapid and more intensive drip losses and the meat becomes exudative and soft. The intensive pH decrease also results more intensive myoglobin destruction resulting pale colour. These meats show higher cooking and frying water losses. Because of these visible changes these meats are called PSE (pale, soft, exudative) meats. Especially some porcine varieties and chicken and turkey tend to show these characteristics. The meats with slow pH decrease show opposite properties; the low amount of glycogen results low amount of lactic acid, therefore minimal protein dehydration and minimal water losses. The surface of these meats seem to be dry and its texture is rather firm. This meat type is called DFD (dark, form, dry). The disadvantage of this kind of meat is the exposure to microbiological spoilage, due to the higher end-pH and the lower lactic acid concentration in the tissues. 2. Quality and quality analysis of meat 81 Created by XMLmind XSL-FO Converter. 12. Determination of meat quality I. (general considerations) The water holding capacity is the ability of meat to hold the original or added water content against external effect, e.g. pressure, centrifugation, heat treatment. The meats with normal quality the water holding capacity is minimal (1 to 4%), this value for PSE meats is higher (5 to 10%). About 5 to 15% of total water content of meat is intercellular water, 85 to 95% is intracellular water, and about 80% of which is immobile, fixed in filaments and the areas between the filaments and only about 2 to 5% is chemically bonded water content. The reasons for water loss are that the area between the filaments decrease what leads to that the water forced out to the closing structure of actomyosin and the proteins of myofibril dehydrate. The pressed out water moves to sarcoplasm, to the interfibrillar space, then to the surface, containing sarcoplasm proteins. Its protein content result decrease in nutritional value but its microbiological importance is much higher; the microbes are grown on these substances rapidly resulting rapid spoilage, especially when the pH decrease is not so rapid. The water losses can be characterized by many ways, depending on practical considerations. The drip loss means that water loss what can be measured without any external forces, just because of the own mass and structure of meat. The thawing loss is that water loss what can be measured after freezing and thawing without applying any external forces. The cooking loss is the water loss can be measured after cooking either with or without the application of external forces. The frying loss is the water loss can be measured after frying. The expressible juice content means that water loss what can be measured after the application of external forces, such as pressing, centrifugation or suction. For the determination of water losses the pressure method is the oldest one. Relatively low mass of sample (1 to 2 g) is placed between filter papers and pressed by 3500 kPa for 3 to 5 minutes. The loss can be measured by weighting or estimated by the determination of wetting area. It also can be determined by centrifugation with centrifugation force from 5000 to 40000g. The drip loss is most commonly determined by the simple principle that a slice of meat is placed on a grate, left alone for 1 to 3 days closed hermetically. The drip loss is the ratio of mass decrease during the test. Another method is when a larger meat piece hanged up in a plastic bag for a specific time. The amount of cooking loss depends on the mass and shape of meat piece and their ratio, the cooking temperature and the speed of warming up, therefore these parameters have to be unified and standardized. The shape of meat is cylinder or rod, the cooking temperature is 75 to 100°C, its time is 50 to 60 minutes or lasts until the meat reaches 100°C as core temperature. The meat is cooled down and rested for a specified time before weighting. The colour of meat is influenced by the colloid chemical properties of meat, the concentration and chemical status of pigments (myoglobin and derivatives). The colour of meat is influenced by the species and the variety, age (the myoglobin content increases and the water content decreases by age resulting deepening in colour), foraging and breeding technology (the animals fed with green forage and hay have darker meat usually, the freerange animals have more myoglobin content due to the more movement), the kind of meat and part of the muscle (white or red meat). The hue is determined by the type of meat; the PDE meats have lighter hue due to the lower pH value and the oxidation of myoglobin is also changes the purple-red colour to cherry-red. The meat colour is also influenced by the pressure, temperature, drying, salting and traces of metals. The brightness is the reflexion of light from the surface of meat and it is in strong connection with its colloid and water status. The wet surface of PSE meat reflects the most of light resulting higher brightness, but the surface of DFD meat has no free water content resulting higher light absorbiton. The colour of meat can be determined by the measurement of total pigment content when the myoglobin, hemoglobin and their derivatives are extracted by organic extractant and their concentration is determined by spectrophotometer. The colour also can be determined by L x a x b colour system on a fresh cut system. The quantifying of hue, saturation and brightness values is also an accepted objective measurement method. The stability of colour, that is the ability to keep its colour during storage is also important and can improved by packing in vacuum and modified atmosphere and using antioxidants. The third physical parameter of meat is its texture. It is partially in connection with the water loss, but the rigor and the proteolitic processes after the rigor are the most important influencing factors. The normal meat is elastic, but the PSE meat is soft and the DFD is firm as it is marked in the name of type. The increase of firmness is detectable during rigor and can be measured by penetrometer (rigorometer). The highly elongated or shortened muscles remain soft or firm after the rigor, respectively. The evaluation of meat texture is done by the Warner-Bratzler shear blade most commonly. For the analysis, fresh or processed (cooked, roasted) meat sample can be used, as this test can be used for the analysis of fresh meat and the effect of different processing methods and their parameters can be also compared and quantified. Most often 5 cylinders from a sample are cut with 0.5 or 1 inch diameter by a sampler, then the specially 82 Created by XMLmind XSL-FO Converter. 12. Determination of meat quality I. (general considerations) designed cutter cut in half with a predefined speed. The resistance of meat is measured during the tesz and the highest counterforce value is the result of the test. The smaller cutting force means more tender meat. The meat shows shortening during the post mortem processes in some cases. It can be cold shortening or rigor shortening, based on its reason In the case of cold shortening the general reason is the rapid cooling as the movement of calcium ions slows down due to the slowing down of Ca-pump and the Ca remains in myofibrils and results large contraction with the present ATP. The cold shortened meat remains rigid, permanently chewy and exudative. The melting rigor is similar to this. The rapid freezing holds back calcium but the membranes damaged and after melting the calcium outflows resulting rapid contraction and using up energy reserves. Shortening occurs in warm meats too, this is the rigor shortening. The lowest shortening can be experienced at 15°C. Several factors influence it, mostly the effects of temperature, pH, stretching of muscle, amount of glycogen and macroerg phosphates and speed of enzymatic processes are evaluated. The shortening increases the rate of exudation. 3. Quality of fat The most important quality parameters of adeps are the firmness, freezing point and thickness. The firmness changes in the cross-section of adeps; the most firm part is the one under the skin. The increasing thickness decreases the firmness, but the amount of connective tissues also influences is. The freezing point refers to the degree of unsaturation; the lower freezing point means higher ratio of unsaturated fatty acids. The less thick adeps has higher water content. Marbling is very important to evaluate. It decreases the drip and cooking losses, improves its tenderness and juiciness. 2 to 3% of marbling is ideal considering the physical properties of meat. The assessment of tallow is different depending on the consumer’s demands; the optimal thickness for cattle meat in countries where steak is commonly consumed is higher, from 5 to 9mm, but in Hungary it is much lower. The tallow protects meat against drying. The following methods are the most frequent methods of meat quality evaluation: • Physiological parameters (pH, metabolite concentrations, temperature, hormone and enzyme levels) • Electrical changes (conductivity, dielectric constant) • Colloid chemical properties (solubility of proteins, rate of denaturation) • Physical properties (colour, exutability, marbling, chewiness, odor, …) • Optical properties • Nuclear magnetic resonance (water holding capacity) • Measurement of boiling losses • Cutting force • Chemical composition The chemical composition of meat is depends on the species, variety, age, conditions of husbandry, slaughter, meat type and composition and other external factors. The water contents of meats range from 55 to 75% in general. The meats of younger animals and the ones poorest in lipids have higher water content. The protein content is between 15 and 22% and its two-thirds part is the proteins of sarcoplasm, about one-fourth part is the proteins of sarcolemma and about 10% is the proteins of connective tissues. The protein content is higher in the meats with low fat content and their digestibility is higher in the case of young animals. The amino acid demands of human body are fulfilled by the meat consumption in adequate amount. The fat content is the storage of vitamins and flavouring substances. Its concentration is between 1 and 45%; the meats with lower than 1.5% fat content are found to be too dry. The meats of mammals are rich in saturated fatty acids, but the marine fish meats are rich source of unsaturated fatty acids. The carbohydrate contents of meats are low (less than 1%) and the concentrations of mineral elements are about 1% too. They are rich in iron, zinc, manganese, copper and selenium and the utilizations of minerals are better comparing to other staple foods. The chemical composition of different meat kinds can be found in Table 13. 83 Created by XMLmind XSL-FO Converter. 12. Determination of meat quality I. (general considerations) 12.1. táblázat - Table 13.: Chemical composition of different meat kinds (Heinz and Hautzinger, 2007) Water Protein Fat Ash Calories / 100g Beef (lean) 75.0 22.3 1.8 1.2 116 Beef carcass 54.7 16.5 28.0 0.8 323 Pork (lean) 75.1 22.8 1.2 1.0 112 Pork carcass 41.1 11.2 47.0 0.6 472 Veal (lean) 76.4 21.3 0.8 1.2 98 Chicken 75.0 22.8 0.9 1.2 105 Venison (deer) 75.7 21.4 1.3 1.2 103 Beef fat (subcutaneous) 4.0 1.5 94.0 0.1 854 Pork fat (back fat) 7.7 2.9 88.7 0.7 812 The quality of meat is influenced by the species, variety and sex. The meat of male intact porcine contains a substance (5 α androstenone) causing unpleasant odor for the product and makes it unacceptable. The susceptibility to PSE and DFD meat quality is strongly influenced by the genotype but environmental effects (transport, resting, stunning, etc.) also play role in their development. The forage influences the ratios of main chemical components but also its elements, e.g. the higher vitamin E content of forage reduces the oxidation spoilages of meat and the fatty acid composition of feedstuffs influences the fatty acid composition of meat. The circumstances of slaughter determine the hygienic properties of product. 84 Created by XMLmind XSL-FO Converter. 13. fejezet - 13. Determination of meat quality II. Qualification of bovine, ovine, porcine, poultry and seafood) 1. General hygienic parameters of slaughter The quality control of primary meat production has general elements obligatory for all kinds of animals. These general requirements define the hygienic expectations on the animals, their handling and the slaughterhouse. The aim of these regulations is creating proper conditions for food safety. The uniform meat quality inspection has three elements: 1. Inspection of animal health in lairage (ante mortem I) The inspection in lairage has to include • the inspection of documentation covering the entire food chain • the sources and compositions of feedstuffs • time and used products of medicinal veterinary treatments and the withdrawal period • presence of diseases may influence human health • results of diagnostic evaluations • records of previous inspections • environmental protection relations • monitoring the health status of the population, to determine whether: • the animals show symptoms of diseases or conditions what caused by food-borne pathogens and these pathogens may influence human health by the handling, treatment or consumption of meat • their behavior refers to the occurrence of disease, which may make their meat unsuitable for consumption • there is any signal that the bodies of animals contains forbidden substances or significant amounts of chemical residues and physical impurities. 2. Inspection of animal health in slaughterhouse (ante mortem II) The control of presence of general personnel and technical conditions is the basis of inspection in slaughterhouse (ramps for guiding animals, suitable place and tools for medical examination and natural illumination for the test, means of transport of injured animals and animal originated wastes, marking tools and trained assistance. The inspection of animals covers: • the inspection of travel documents (veterinary certificates, identification marks) • the visual inspection of animals on the vehicle (infectious disease, toxicity) and during moving: • the posture, movement and breathing of animals • the integument (skin, hair, wool) state • the visible mucous membranes and their surroundings • the internal temperature of animals 85 Created by XMLmind XSL-FO Converter. 13. Determination of meat quality II. Qualification of bovine, ovine, porcine, poultry and seafood) The slaughter (or the entry of animals to the slaughterhouse) must be denied when • the animals are not sufficiently clean • some of them died during transfer • their identification is not sure • there is any suspect for zoonotic disease threat to either animals or humans • the declaration of producer or authority on the veterinary status is missing or inadequate 3. Hygiene inspection of meat after slaughter (post mortem) The hygiene inspection of meat after slaughter must be done by veterinarian. Based on the certificate of an official risk analysis it may be ignored. For the inspection all parts of the slaughtered animal must be presented or provided. The manual inspection includes the detection of visible abnormalities by the inspection of selected organs (views, smell, touch and incisions). Based on the result of the post mortem evaluation the meat can be considered as • suitable for human consumption • safe and suitable for human consumption • suitable for human consumption with lower nutritional or organoleptic value • suitable for human consumption after the application of a prescribed process • conditionally suitable for human consumption • not suitable for human consumption but able to use for other purpose • held on suspicion of being unsafe or unsuitable, pending the outcome of further procedures and/or tests • unsafe for human consumption and requiring condemnation and destruction The disorders of meat can be stuffy odor (as a result of chemical processes of abnormal post mortem changes in muscles), putrefaction (bacterial degradation), colour disorders (yellow colour disorders due to the carotenoids of forage, icterus caused by high bilirubin, black colour disorders because of melanin, brown-black colouring of bones due to the porphyrin), smell and taste disorders (alimentary disorders due the forage, sexual smells, vital smell disorders caused by diseases of organs, post mortem smell disorders caused by foreign odors during the handling of meat). The texture of meat can be evaluated by organoleptically and microscopically. The fields of tests are the status of connective tissue under the skin, the leakage of whey on the pleura, the ganglions, the interior of bones, size of organs and the amount of dripping loss. The disorders of fatty tissues can be physical and chemical (rancidity). The inspected meat has to be marked. 2. Sources of quality requirements on meat The UNECE have detailed requirements of meat quality for bovine, veal, ovine, porcine, caprine, chicken, duck, turkey, goose, horse and llama/alpaca. Similarly to the standards on fruits and vegetables, these standards contain the minimum requirements on the products (carcasses and cut parts), trade categories for refrigeration, age and sex of animals, production systems (e.g. intensive, organic, etc.), feeding system (e.g. grain, forage, etc.), slaughter system (e.g. conventional, kosher, etc.), limits for fat coverage and quality systems. The summary of minimum requirements of UNECE on bovine, ovine, porcine, turkey and chicken carcasses and cuts can be seen in Table 14. 13.1. táblázat - Table 14.:Minimum requirements on meat quality (UNECE standards: ECE/TRADE/326, ECE/TRADE/308, ECE/TRADE/369, ECE/TRADE/358, ECE/TRADE/355) 86 Created by XMLmind XSL-FO Converter. 13. Determination of meat quality II. Qualification of bovine, ovine, porcine, poultry and seafood) bovine ovine porcine turkey chicken Intact, taking into account the presentation Free from visible blood clots, or bone dust. Free from any visible foreign matter (e.g. dirt, glass, wood, rubber metal particles – depending on species) Free of offensive odors. Free of foreign odors Free of fecal contamination Free of obtrusive bloodstains Free of improper bleeding Free of unspecified protruding or broken bones. Free of viscera, trachea, esophagus, mature reproductive organs, and lungs Free of contusions having a material impact on the product Practically free haemorrhaging Free of spinal cord (except for whole unsplit carcasses) Free of gall discoloration of feathers and Free of freezer-burn 3. Qualification of bovine meat The quality requirements on bovine meat can be found in the EC No 1249/2008 Commission Regulation. It is also known as SEUROP system as the different categories based on the development of carcass profile. It defines five categories for bovine carcass; carcasses of uncastrated male animals less than 2 years old; carcasses of other uncastrated male animals, carcasses of castrated male animals, carcasses of female animals that have calved and carcasses of other female animals. In Hungary slaughter calf, slaughter heifer, slaughter bull, juvenile and adult cattle, slaughter cow, slaughter stirk and ox categories were declared, but the decree no. 76 of 2003. (VII. 4.) FVM creates other trade categories based on gender and weight: • bovine less than 80 kg (formerly slaughter calf) • bovine between 80 and 160 kg (formerly slaughter calf) • bovine between 16 and 300 kg (formerly slaughter heifer, slaughter bull, juvenile and adult, slaughter stirk) • heifer, not calved, higher than 300 kg (formerly slaughter heifer) • cow for slaughter (formerly slaughter cow) • other bovine for slaughter (formerly slaughter beef cattle, slaughter bull, juvenile and adult, slaughter stirk and slaughter ox) Besides other categories are also used in practice. White meat calf is the young, 20 to 22 weaks old animal with 180 to 200 kg weight what has white meat colour due to the milk and milk substitute based forage. Baby beef is the 10 to 12 months old animal with 350 to 400 kg weight. In some cases young cow is the female adult has calved one time. The evaluation of development of carcass profiles and its essential parts is the second important qualification field of the EC Regulation. There are six categories specified by the letters of SEUROP where S means superior quality and R does the poor quality. It considers the form of carcass profile and the three most important body parts (round, back and shoulder). The classification criteria can be found in Table 15. 87 Created by XMLmind XSL-FO Converter. 13. Determination of meat quality II. Qualification of bovine, ovine, porcine, poultry and seafood) 13.2. táblázat - Table 15.: SEUROP classification criteria for bovine by the development of carcass (EC No. 8/1994; EC No 1249/2008; Ostojić-Andrić et al., 2012) Conformation S - Superior EExceptional U- Very good Profiles - all profiles all profiles profiles on the profiles on the profiles extremely convex to whole convex whole straight straight convex super-convex concave - Round very highly rounded doublemuscled visibly separated seams Topside spreads very markedly over the symphysis topside spreads markedly over the symphysis rounded Topside spreads over the symphysis Back very wide and very thick, up to the shoulder Rump very rounded wide and very thick, up to the shoulder Rump very rounded wide and thick, still thick but average narrow with up to the less wide at thickness to bones visible shoulder the shoulder lacking thickness Rump rounded Rump: straight profile Shoulder very highly very rounded rounded rounded R- Good O- Fair P- Poor all profiles to concave to very concave wellaverage poorly developed development developed Topside and to lacking rump are development slightly rounded fairly well- average developed development to almost flat flat with bones visible The third part of bovine qualification is the fat cover outside of the carcass (under the skin) and in the thoracic cavity. The classes are defined from 1 to 5 where 1 means the lack of fat in the thoracic cavity and there is only a very low amount of fat cover and the carcasses with heavy fat coverage and deposits are marked with 5. The detailed groups can be found in table 16. 13.3. táblázat - Table 16.: SEUROP classification criteria for bovine by fat cover (EC No. 8/1994; EC No 1249/2008; Ostojić-Andrić et al., 2012) Fat cover 1-Low 2- Slight Outside of the None up to low Slight fat cover, carcase fat cover flesh visible almost everywhere 3- Average 4- High 5-Very high Flesh with the exception of the round and shoulder, almost everywhere covered with fat Flesh covered with fat, but on the round and shoulder still partly visible – The seams of fat on the round are prominent Entire carcase covered with fat – The round is almost completely covered with fat, so that the seams of fat are no longer clearly visible In the thoracic No fat within the Within the Slight deposits Some distinctive Heavy deposits in cavity thoracic cavity thoracic cavity of fat in the fat deposits in the the thoracic the muscle is thoracic thoracic cavity – cavity – Within 88 Created by XMLmind XSL-FO Converter. 13. Determination of meat quality II. Qualification of bovine, ovine, porcine, poultry and seafood) clearly visible cavity Within Within the between the ribs the thoracic thoracic cavity cavity the the muscle muscle is still between the ribs visible between may be infiltrated the ribs with fat the thoracic cavity the muscle between the ribs is infiltrated with fat There are other former and newer methods are available for the estimation or the determination of meat quality. The “butcher catches” are the most ancient qualification method of slaughter value of bovine; the trained butcher estimated it by the amount of fatdeposits in the subcutaneous connectivetissues(touched the shoulders, ribs, scrotum, around the heart,, perineum, underbelly, throat) and the intermuscular fat content (touched the transverse processes of lumbar vertebra). The scaling is the measurement of the circumferences or sizes of the different body parts and its visual way is the condition inspection. The diameter and volume of fat cells refers to the ratio of tallow. Ultrasonic methods are also used for the estimation of muscle parts and the thickness of subcutaneous tallow layer. The creatinine content of urea also found to be correlated to the amount of meat. After slaughter the analysis of cross-section of steak can be used for the valuation of the tissue composition of the whole body. Similarly the separation of different tissues (meat, tallow and bone) their ratios can be determined and projected to the whole body. The mass and size of bones are also predicts accurately the mass of meat. 4. Qualification of porcine meat The quality of porcine meat also can be valued by its composition (mass of muscle, fat and bone tissues and their ratio) and by the meat quality (normal, PSE and DFD). Although the frequency of PSE quality is much higher in the case of porcine than in the case of other species the quality of carcass is determined by the ratio of lean meat. The fat content of porcine body strongly influenced by the variety, age and conditions of husbandry and the consumer generally requires low fat content and the marbling is not favorable, but required in some cases (easy pork or broiler pig). The quality requirements on porcine meat applied in the European Union can be found in the EC No 1234/2007 Commission Regulation. The Decree No. 136 of 2011 (XII.22.) of the Ministry of Rural Development adapted these regulations in the Hungarian practice. These regulations do not word requirements on age, sex and other parameters but give limit values for pig meat qualification from 50 to 120 kg warm weight. In contrast, the UNECE ECE/TRADE/369 standard defines categories by sex and age, production system and feeding system. Practically the general trade classes are the following: • suckling pig: fed on the milk of its mother and slaughtered in the 4th to 6th week (5 to 15 kg) seasonally for roasting • light pork: young porcine slaughtered with 40 to 60 kg living weight for special purposes (substituting lamb meat in several countries) • heavy pork: porcine slaughtered with 60 to 80 kg living weight and sold as fresh extra lean meat. • bacon pig: porcine with white bristles and pigment-free skin slaughtered with 80 to 90 kg living weight giving extra quality meat or slaughtered with 100 to 110 kg living weight and the ratio of lean meat has to be exceed 50% • lean pork: porcine with slight pinguefaction slaughtered with 110 to 120 kg living weight • pigs with industrial meat: obese lean pork slaughtered with 130 to 150 kg living weight • lard pig: high fat content porcine varieties The quality of carcass is determined by the ratio of lean meat and cold carcass using the marks of the SEUROP qualifying system. When the lean meat as percentage of carcass weight is higher than 60% the carcasses is classified into the S group, when it is between 55 and 60% it is classified into E group and further 5% decreases are the limits for the U, R, O and P group, therefore the carcasses with less than 40% lean meat content is in the P group (Table 17.). 89 Created by XMLmind XSL-FO Converter. 13. Determination of meat quality II. Qualification of bovine, ovine, porcine, poultry and seafood) 13.4. táblázat - Table 17.:SEUROP classification criteria for porcine (EC No 1234/2007 Commission Regulation; Decree No. 136 of 2011 (XII.22.) of the Ministry of Rural Development) Category Lean meat as percentage of carcass weight S 60 % or more E 55 % or more, but less than 60 % U 50 % or more, but less than 55 % R 45 % or more, but less than 50 % O 40 % or more, but less than 45 % P less than 40 % The amount of lean meat can be estimated using approved measuring devices (penetration test or ultrasonic evaluation) and regularly revised regression equations. The parameters used for estimation are the diameter of chop (back-fat and dorsal muscle thickness) between the second and third last ribs, measured at 6 or 7 cm off the midline of the split carcass (depending on the used equipment) or the minimal fat depth (including rind) over the Musculus gluteus medius and the minimal muscle depth between the anterior extremity of the Musculus gluteus medius and the dorsal. The regulations contain the actualized regression equations. The quality of porcine meat can be evaluated by other practical, industrial or scientific methods. Halothane probe and serum enzyme tests are used for forecasting the non-normal meat quality. The ultrasonic probe is used for the ante mortem evaluation of meat quality, size and location of organs, thickness and volume of adeps even during moving of the animal. The infrared probe is also suitable to mapping the living body. Computer tomography, magnetic resonance and other methods used primarily in human medicine are also used in research and improvement of practical methods. After slaughter the separation of skin, adeps and meat and boning are the most accurate way to determine the ratio of lean meat and fatty adeps. Their ratio can be estimated by the electric conductivity of the prepared meat. 5. Qualification of ovine meat The official classification system in the European Union is laid down in the Commission Regulation (EC) No 22/2008: It lays down requirements for the sheep under 12 months (lambs) and other ones for marking. The ECE/TRADE/308 UNECE standard defines more aspects for classification; sex and age (young lamb, lamb, hogget, mutton, ewe mutton, wether mutton and ram), production system, feeding system, slaughter system and fat thickness (peeled carcass or covered with fat and categories draw up by 3 mm increase up to 15 mm). The EC 22/2008 regulation classifies the ovine carcass into SEUROP system. Similarly to the classification of bovine, the appearance of main profiles are the basis for the SEUROP tags and the fat cover determined by visual evaluation ranks them from classes 1 to 5. For example, the requirements of S (superior) category are that the hindquarter is extremely muscled and its profiles are extremely convex; the back is also extremely convex, wide and thick and the shoulder is extremely convex and thick. The 1st fat cover class requires that external fat cover, fat on kidneys and between ribs cannot be visible or only in traces. The other classes for meat quality define less thick to narrow meat profiles and the classes for fat cover define increasing fat layer thickness and coverage for different body parts and organs. 6. Classification of poultry meat The slaughter poultry is the poultry no older than 10 weeks reared intensively or younger than 20 weeks reared extensively. Besides more categories are present in practice by size: mini broilers are from 700 to 900 g, small broilers are from 1000 to 1300 g, grill broilers are from 1400 to 1700 g, normal broilers are from 1800 to 2500 g and maxi broilers have their weights higher than 2500 g. The roaster is a special category; they are reared to 90 Created by XMLmind XSL-FO Converter. 13. Determination of meat quality II. Qualification of bovine, ovine, porcine, poultry and seafood) 3000 g for the 9-12th week old age. The capons are the neutered cocks older than 140 days. The EEC No 1538/91 Commission Regulation defines the official categories by sex and age for domestic fowl, turkey, duck, geese and guinea fowl. The categories for domestic fowl are: chicken is that young animal of which the tip of the sternum is flexible; the tip of sternum is rigid for the cock, hen, casserole or boiling fowl. The capon is the male what is older than 140 days, castrated before reaching sexual maturity and fattened for minimum 77 days. The coquelets are those chickens which have less than 650 g carcass weight without giblets, head and feet. The chicken of 650 g to 750 g are the poussins if the age less than 28 days. The young cock is that male chicken of laying strains in which the tip of sternum is rigid but not completely ossified and slaughtered younger than 90 days. The ECE/TRADE/355 UNECE standard defines other categories for chickens by age and sex; the very young chickens are the ones less than 4 weeks old, the young chickens less than 12 weeks old and have flexible sternum tip, the roasters are also less than 12 weeks old but the tip of their sternum is not so flexible than for the previous category, capons I and II are the neutered very young and young chickens. The egg-laying hens and breeding hens and roosters are older than 10 months. The UNECE standard defines other groups by boning, presence and colour of skin, production, feeding, slaughter and chilling system and applied anti-microbial treatments. The poultry carcass or cuts can be classified into A and B quality groups by the EEC No 1538/91. Both classes have to fulfil the minimum requirements: they have to be intact, clean, free from foreign matter, dirt, blood, foreign smell, broken bones, severe contusions and larger visible bloodstains. The categories are defined by the conformation, the development of muscles and fat content of body parts and the amount of feather, stub and hair remains and other specified damages. From the consumer’s demands the colour of skin is important; it can be yellowish or whitish, determined by its pigment content and nutrition. The colour of meat is typical for the species; for chicken the lighter for turkey the darker colour is expected. 7. Requirements on fish and seafood In the case of the live fish, their eggs and gametes the 2003/858/EC lists the requirements. It is required to be originated from specified areas (declared by species), fit the hygienic, labeling and other general requirements, and the fishes have to be transported in such conditions that do not influence their health status. The known diseases to be evaluate and susceptible species are also listed. On the other hand, the microbiological status also can be examined and characterized by total viable counts or aerobic plate counts. The lots have to be accompanied by the catch certification. Due to the exposure of seafood to the inorganic and organic contaminants in the environment, the maximum allowed concentrations of some kinds of contaminants are also declared. The 1881/2006/EC and 420/2011/EU prescribe limit values for mercury, lead, cadmium, dioxins, PCBs and polycyclic aromatic hydrocarbons in the different seafood. The fishes can be classified into Extra, A and B categories based on their freshness by following the regulations of Codex Alimentarius, based on the status of skin, eye and gill, colour of different organs, texture of flesh, spine and peritoneum and odour of gill, skin and abdomen. Size is the another classification aspect, the categories are declared by species. 91 Created by XMLmind XSL-FO Converter. 14. fejezet - 14. Requirements and properties of milk and egg 1. Quality of milk The requirements on milk quality are different for the different levels of processing chains. Based on the definition of Codex Alimentarius Hungaricus, raw milk is the product what milked from cow, sheep or goat, did not heated above 40°C or treated equivalently and did not distracted or supplemented by any compounds. Raw milk can be classified to made for fresh consumption or for processing. Its quality can be evaluated on the farm after milking or at the acceptance places and the typical quality parameters are its wholesomeness, bacteriologic and chemical properties. The second product group specified by the Codex Alimentarius Hungaricus is the pasteurized milk and chemical and microbiological requirements are set. The third group is the fermented milk products and limit values for chemical and microbiological (pathogens and prebiotics) quality parameters are listed. Maintain the hygienic conditions and the cooling chain in the milk production is the most important quality related issues. Milk can be produced on those farms what are registered and controlled by the competent veterinary authority. The livestock and the circumstances of milking and milk handling have to be done under controlled hygienic conditions. The milk must be cooled down to 4 to 65°C within 2 hours and the possible storage time depends on the temperature of milk; on 4°C it can be stored for 48 hours, on 6°C for 24 hours, on 8°C for 16 hours. The raw milk for fresh consumption has to be stored and distributed only within 2 hours from milking. The quality parameters of milk can be classified into physical, chemical and biological groups. The main physical properties of milk are: • density: the average density of milk is between 1.029 and 1.033 g/cm3, depending on the composition (fat vs. other compounds). The lower value refers to water addition, the higher values for refused milk. • freezing point: the normal freezing point of fresh milk is -0.52°C. The water addition results higher value. • viscosity: it is in connection with the mouthfeel and flavor release. The viscosity of milk is influenced by the fat content and the size of protein micelles and fat dispersion. • colour: the colour of milk is white or yellowish-white; the forage and health status of animal influence it. The naturally separated fat layer has to be easily dispersible. • odor: it has to be free from foreign odors. • taste: it has to be typical of milk, sweetish and free from foreign taste. The most important chemical parameters of milk are the pH or acidity and the concentrations of chemical compounds. The acidity is a simple parameter for the characterization of freshness as the lactic acid bacteria form the lactose content of milk to lactic acid resulting increase in its acidity. The pH of milk is between 6.6 and 6.75, characterizes the actual acidity of milk. The potential acidity or titratable acidity (Soxhlet-Henkel, °SH) shows the amount of H+ ions of the whole reactive compounds with a typical value from 6.3 to 6.6 for the fresh milk. The SH acidity shows that how many NaOH in ml is required to neutralize 100 ml of milk in the presence of 2% alcoholic phenolphthalein indicator solution. The low (less than 5 SH°) refers to mastitis and the milk with high SH acidity (higher than 8 SH°) resulting sour taste and coagulation of colloids of milk. The Thörner acidity is also used. It shows that how much NaOH solution is required for raising the pH of the milk to about 8.3. The biologic properties of milk are determined by the number of somatic cells (the cells originated from blood and mammary glands and have nucleus) and the present microbes. The milk itself also has biologic properties; on one hand it is suitable for fermentation, on the other hand it has protection ability due its bactericid and bacteriostatic ability (lactoferrin and other proteins, enzymes, immunoglobulins and granulocytes). 92 Created by XMLmind XSL-FO Converter. 14. Requirements and properties of milk and egg The chemical compositions of milks of different species are different. The water content is the highest value, it is between 80 and 88%; the lowest values are valid for the most of bovine and goat, the highest values are for sheep and the bovine with high-fat-content milk (e.g. Jersey). Nutritionally the protein content has the highest impact; it is about 3.1 to 3.7 for cattle; the protein content of sheep milk is almost double, but the one of goat milk is slightly less. The lactose is the dominant carbohydrate of milk; the cattle milk shows the highest values with about 5% and the milks of goat and sheep have a 1% less value. The fat content is very important due to its importance in the determination of price; the milk of goat and cattle produces consumer quality milk contain about 3.7% fat, the cattle varieties produce industrial milk has higher than 4.5% fat content and the sheep milk has higher than 7% fat content (Table 18.). 14.1. táblázat - Table 18.: Chemical composition of milk Cattle1 Sheep2 Goat2 Holstein Jersey Total solids, % 12.4 14.6 17.95 12.48 Fat, % 3.7 5.1 7.62 3.8 Solids-not-fat, % 8.7 9.5 10.33 8.68 Protein, % 3.1 3.7 6.21 2.9 Lactose, % 4.9 5.0 3.7 4.08 Other, % 0.7 0.8 Source: 1http://www.thecattlesite.com, 2 Jandal, 1996 The nitrogen containing compounds of milk are mostly proteins and amino acids. A part of the proteins are phosphoproteins and can be precipitated by organic or inorganic acid addition; this part is called casein. Its ratio is about 80% in the total protein content of cattle milk and it contains all the essential amino acids. It present as small spheres forming the fine disperse system making suspension in the milk and giving the white colour of it. The non precipitable parts of proteins are the whey and its components are the lacto-albumins and lactoglobulins. The amount of free amino acids is low as well as the other nitrogen compounds (e.g. adenine, creatinine, xanthine, intermediate metabolites, etc.). The main carbohydrate of milk is the lactose, disaccharide of a sucrose and a galactose molecule. The quality parameter shows the highest variability by the circumstances of husbandry is the fat content. The fat is present as emulsion in the milk formed as small (1-20 µm) spheres forming the rough disperse system of milk. The size of fat globules is higher for the sheep milk resulting stronger tendency to skimming while the size of fat globules in goat milk is much lower than the ones of cattle milk. It is built mainly from triglycerides, its two-third part is saturated and one-third is unsaturated (partially polyunsaturated). Beside lipids lipoids are also present in the milk having emulsifying effect on the disperse system. The element and vitamin contents of milk are also important elements of quality. The qualification of milk is based on the physical, chemical, hygienic and sensory parameters. The density of raw milk can be determined easily by lacto densitometer reporting rapid information whether the milk contains added water. The requirement of Codex Alimentarius Hungaricus is 1.028 g/cm3. The determination of freezing point is also a rapid and inexpensive method for this purpose; it should not exceed -0.52°C. The composition of milk is required to fit to the natural composition. Its protein content has to be higher than 2.9% (Table 19.). 14.2. táblázat - Table 19.: Physical and chemical requirements on the raw cattle milk (Codex Alimentarius Hungaricus 2-51) Parameter Requirement 93 Created by XMLmind XSL-FO Converter. 14. Requirements and properties of milk and egg Amounts of components Fit to the natural composition Protein content, at least 2.9% (m/m) - N content x 6.38 Density (20°C), at least 1.028 g/cm3 Freezing point, at most -0.520 °C The Codex Alimentarius Hungaricus lists limit values for microbes and somatic cell number as hygienic requirements. Different maximum allowed number for microbes is required for milk for fresh consumption and industrial use (Table 20.). The requirements on somatic cell number and Staphylococcus aureus are the same for both use and Salmonella and other pathogens are also required to determine for milk for fresh consumption. Inhibitors should be less than the detection limit for both kinds. 14.3. táblázat - Table 20.: Biological requirements on the raw cattle milk (Codex Alimentarius Hungaricus 2-51) Parameter Raw milk for fresh consumption Raw milk for industrial use Microbe number, CFU/cm3 ≤ 50 000 ≤ 100 000 Somatic cell number, CFU/cm3 ≤ ≤ Inhibitors below the detection limit below the detection limit Staphylococcus number/cm3 Salmonella ssp, number/cm3 aureus, n=5, c=2, m=100, M=500 n=5, negative in 25g Other pathogens and/or their toxins below the individual limit values n=5, c=2, m=500, M=2000 - The requirements on the raw sheep and goat milk is the. The compositions of these milks have to fit to the natural composition. The requirement on freezing point is the same as it was for cattle milk similarly to the ones on inhibitor content and Staphylococcus aureus, but the allowed presence of microbes is much higher (the microbe number has to be less than 1 500 000). The sensory requirements are also the same for all raw milk kinds. Their colour has to be white or yellowishwhite, they have to be homogeneous, free from visible alterations, and the skim has to be dispersible by mixing. The odor of milk should be characteristic, free from foreign odors. The taste also has to be characteristic, slightly sweet, full and free from extraneous flavours. Other requirements are present for the pasteurized milk products (Table 21.). The general requirements are partially the same to those of raw milk (freezing point, density and protein content), but the fat content has to fit for the type of product; it has to be less than 0.5% for skim milk, between 1.5 and 1.8 for skimmed milk, 2.7 and 2.9 for semi-skimmed milk and higher than 3.5 for whole milk. The type of pasteurization has to be as it is indicated in the name. The milk has to be equable, slightly yellowish or ivory, according to the fat content. It has to be homogenious, free from flocculation, sediments and skimming. Skimming is allowed for the not homogenized milk only, what have been heat treated on high temperature, and the skim has to be easily dispersible by mixing. The smell of pasteurized milk has to be characteristic, slightly cooked, pure, and its taste should be characteristic, sweet, mildly or slightly cooked and pure. 14.4. táblázat - Table 21.: Requirememts on the pasteurizated milk products (Codex Alimentarius Hungaricus 2-51) 94 Created by XMLmind XSL-FO Converter. 14. Requirements and properties of milk and egg Parameter fat content Requirement skim milk above 0.5 % (m/m) skimmed milk between 1.5 and 1.8 % (m/m) semi-skimmed milk 2.8 ± 0.1 % (m/m) whole milk at least 3.5 % (m/m) pasteurization according to the name protein content at least 2.9 % (m/m) density 1.028 g/cm3 freezing point -0.520 °C appearance Equable, slightly yellowish or ivory, according to the fat content substance Homogenious, free from flocculation, sediments and skimming. Skimming is allowed for the not homogenized milk, heat treated on high temperature, when the skim is easily dispersible by mixing smell Characteristic, slightly cooked, pure taste Characteristic, sweety, mildly or slightly cooked, pure The definition of fat corrected milk is developed for the allowance of commercial transactions and the valuation and comparison of different animals. This means that the amount of milk is calculated for a standard basis of a milk with 4% fat content. This calculation can be done by the Gaines formula: FCM= 0.4 × mass of milk (kg) + 15 × fat yield of milk (kg) 2. Quality of eggs The eggs in trade sense is eggs of hen in shell suitable for human consumption and industrial food processing except when they are broken, boiled or incubated, however, the industrial egg can be broken or incubated. The four main parts of eggs are the shell, fluid and thick albumen and the yolk from the aspects of qualification (Figure 18.). The shell protects the eggs against external physical and other effects have high dry matter content and its most important chemical component is the calcium. The albumen is separated from it by the shell membrane which is a double pergameneous membrane formed mainly from proteins. There is an air cell between these membranes. The albumen protects the yolk by its chemical composition and the chalazas what fix the position of yolk and help the germinal disc to turn up. The outer part of albumen is the thin albumen poor in mucin while the thick albumen contains a lot of mucin fibers. The high moisture content of albumen is important for maintaining the water balance of eggs. Its protein content is important source of nutrients for the embryo and contains several defensive compounds; for example the heat resistant ovomucoid what hinders the protein destruction enzymes, the ovotransferrin what inhibits the bacterial growth and ovoglobulins what has bactericide and virucide effect. The yolk is formed from concentrically different layers white yolk (about 5% of the total yolk) and yellow yolk. The yolk contains the half of the proteins and all of the lipids of eggs. 95 Created by XMLmind XSL-FO Converter. 14. Requirements and properties of milk and egg 14.1. ábra - Figure 18.: The structure of eggs The chemical compositions of the different part of hen eggs are different (Table 22.). The albumen is nearly 60% if the total eggs in mass. Its moisture content is about 88%, more than 10% its protein content and contains lipids, minerals and other components in less than 1%. The yolk gives about 30% of the mass of eggs and contains almost the total fat content of eggs; its lipid concentration is higher than 30%, its protein content is 16 to 17%. The moisture content of yolk is 48% on average but the moisture content of white yolk is nearly the same to the value of thick albumen. The mineral content of yolk is also higher than the one of albumen. 14.5. táblázat - Table 22.: Chemical composition of eggs (Tanács, 2005) Parameter Yolk Albumen Moisture content 48% 88% Lipid content 31% 0.01 Protein content 16.5 10.6 Mineral content 1.7 0.7% Others 1.9 1.1 The quality of eggs is determined by the sensory and physical properties. The Codex Alimentarius Hungaricus defines three quality categories; class A is the group of fresh eggs, the class B is the group of the second class or preserved eggs and class C is the group of poor quality eggs suitable for industrial use. The 1907/90/EEC regulation defines two classes only; A is the group of fresh and B is the group of industrially processable eggs. The eggs classified into the group A have to be normal shaped, clean and free from damages. The height of air cell should not exceed 6 mm (or 4 mm for fresh eggs). The albumen has to be clean, translucent, gelatinous and free from foreign matters. The yolk has to be only as a shadow during candling, it should not move away from 96 Created by XMLmind XSL-FO Converter. 14. Requirements and properties of milk and egg the center of egg during rotation. The germinal disc has to be non-detectable and the odor of egg has to be free from foreign odors. The A class eggs can be classified by their masses. The eggs with less than 53g mass is marked with S, the ones with from 53 to 63 g with M, the ones from 60 to 73 g with L and the ones higher than 73 g with XL mark. The mass of egg is influenced by genetic properties, age and feeding of hen, temperature and illumination of poultry house. The other poultry species has different egg mass on average; it is between 80 and 90 g for turkey and duck but 150 to 160 g for goose. The required mass for hen hatching eggs is between 58 and 62 g. Beside mass the shape of eggs is also important due to its influence on the transportability and exposure to mechanical defects. A 1.23 to 1.40 ratio of longitudinal and cross diameters is the required. The colour of shell can be white or brown and influences the consumer’s acceptance; the darker shell colour is associated with higher nutritional value for several consumers although difference in chemical composition does not proved, the colour of shell is only typical of the variety. The colour of eggs in a lot should be homogeneous. The thickness and hardness of shell are also important due to the transportability and processability; shell has to be resistant to mechanical stresses of manipulation. It is strongly influenced by the forage of hens, especially its element composition. The most frequent shell defects of shell followings: • Cracks: both the shell and the shell membrane are damaged and the content of egg is exposed to contamination. Its main causes are inadequate nutrient supply (Ca, D vitamin), heat stress, saline water and the senescence of hens. • Thin-shelled eggs and lack of shell (only shell membrane covers the shell partially or fully) caused by inadequate nutrient supply or disease • Misshaping: deformed shape (spheroidal or long) or small or large in mass casued by disease or inappropriate conditions • Staining: blood staining appears on the shell due to the lack of hygiene or overweight hens • Fly marks: caused by the flies due to inappropriate hygiene • Speckled shell: white or brown spots are appears mainly due to the lack of calcium in forage, disturbance in calcification or shell gland • Microbiological defects The height of thick albumen and the height and colour of yolk refers to the freshness of eggs. Both the heights of thick albumen and yolk decrease by time and the quality of eggs can be characterized by the Haugh unit: where H is the height of albumen in mm and W is the mass of egg in g. Usually the eggs keeps their freshness up to 21 days. The colour of yolk also changes during storage; the substances of yellow yolk diffuses into the white yolk resulting lightning in colour. The most frequent internal egg defects are the followings: • Blood spots: blood spots or lines in the albumen or adhered to the yolk • Meat spots: solid material floats in the albumen • Watery parts: abnormally thin parts in the albumen or yolk • Pale yolk: caused by the age of eggs or the inadequate forage • Microbiological defects • Off-odor and taste 97 Created by XMLmind XSL-FO Converter. 14. Requirements and properties of milk and egg The U.S. grading of eggs defines three categories; AA, A and B. The shell has to be clean, unbroken and normal, slight stains and abnormalities are allowed for B quality group. The size of air cell is defined for all grades. The albumen has to be clear and firm; small blood and meat spots and watery character are allowed only for grade B. The grading has requirements on the outline of yolk (slightly, well or plainly defined for AA, A and B classes, respectively) and declares that it has to be free from defects. It also accepts the dirty but unbroken eggs and the eggs with broken shell but intact shell membranes. The U.S. grades for size are the followings: jumbo is 70 g or higher, extra large is between 65 70 g, large is between 56 and 65 g, medium is between 49 56 g, small is between 42 and 49 g, peewee is from 35 to 42 g. The requirements on hatching egg are different to those of fresh ones. The most important parameters of them are the fertility and hatchability. The fertility of egg is different in the cycle therefore the selection of hens for producing fertile eggs is very important. The hatchability of eggs can be characterized by the ratio of number of hatched chicklings and the number of fertile eggs or the ratio of number of hatched chicklings and the number of eggs placed into the hatchery. The latter one is easier to determine and it can be used better in the circumstances of husbandry. 98 Created by XMLmind XSL-FO Converter. 15. fejezet - 15. Systems for products grading and quality certification laboratories The analytical laboratories can be classified into two main groups based on the acceptance their activities. When the results of the laboratory are used for producing results and data for processing, automation or other internal use the requirements are the suitability, cost efficiency and rapidity. However, when the results will be used for trade operations or official purpose the handling and storing of samples, the measurements, the handling of data and any other direct or indirect activity influencing the result have to be controlled and certified by independent organization. In this case the main requirements are accuracy and reliability with timeliness. To provide this kind of accuracy the laboratories have to introduce and maintain a quality assurance system. The two most important quality assurance systems for laboratories are the followings: • Good Laboratory Practice (GLP): it is developed for the non-clinical research fields to provide uniformity, consistency, reliability quality and reproducibility for the analytical activity considering chemical, physicochemical and toxicological fields. In Hungary 9/2001. (March 30) EüM FVM prescribes its application for analysis and service providing in production of human pharmaceutical, veterinary medicine, plant, pesticide and hazardous substances • ISO/IEC 17025:2001: General requirements for the competence of testing and calibration laboratories, testing and calibration laboratories: it is a standardized system for the quality assurance of any analytical laboratory and can be apply on all analytical fields. As a standard, it can be ulinked to other quality assurance standards and standardizes the content for the documentations. Comparing to the GLP it is a broader, more comprehensive regulatory system „Good Laboratory Practice (GLP) is a quality system concerned with the organisational process and the conditions under which non-clinical health and environmental safety studies are planned, performed, monitored, recorded, archived and reported” (OECD GLP Handbook, 2003). It declares the general rules to control the compliance of requirements of GLP, the site inspections of GLP with a subsequent review of the analyses and the procedures applied in inspections and also prescribes the professional requirements of the execution of GLP inspection. The main regulation fields of GLP are: 1. Resources, containing rules on the organisation, personnel, facilities and equipment The GLP prescribes the minimum number of personnel for the key positions of the laboratory. Every laboratory has to have a head who directs the operative work and strategy of laboratory and a quality manager who maintain the quality assurance. The analytical activity is performed by professional analysts and the role of support staff is also defined. The quality assurance documentation has to contain records on the competence of stuff and the continuous education, its verification and recording is also prescribed for the analytical laboratory. Regarding areas and facilities different requirements on the laboratories and support rooms where the analytical activities take place, the public and the private areas are required considering their sizes, constructions and arrangements as well as the regulation of environmental conditions, such as temperature, relative humidity, vibration-free location, special lighting and radiation protection. Areas have to be free from microbial contamination from the gaseous and dust, cross contamination, overcrowding or other nuisance factors. The fields of regulated equipment are all that devices which used in the laboratory activity. Requirements are defined on their suitability, calibration and maintenance. The equipments used in the analytical activities are: • general-purpose tools: not used in measurement, but its use may influence the measurement (from the cookers, blenders, non-volumetric measurement to the general laboratory heating or ventilation system) • volumetric devices (e.g. volumetric flasks, pipettes, pycnometer, burettes) 99 Created by XMLmind XSL-FO Converter. 15. Systems for products grading and quality certification laboratories • measuring instruments (eg. viscometers, thermometers, clocks, chromatographs, electrochemical measuring instruments, scales) • etalons, thermometers, certified reference material (CRM) • computers and data processing units The use and registration of chemicals are also controlled by GLP. Continuous and maintain accurate records have to be kept both on the quality and the quantity of them. The quality of used chemicals have to meet the requirements in purity, their producers have to be work by EN 29000 or ISO 9000. The chemicals have to be identified individually. The precautions, storage and use instructions have to be followed as well as the procedure of handling of unused, expired chemicals. 2. Characterisation The range of rest items and test systems is very board; animal, plant, chemical, soil and other, therefore the content of regulations different on different test items. In every case the knowledge on the tested item (material) and used chemicals is the most important; their physical, chemical and biological properties and the purpose of analysis jointly determines the storage, handling and safety requirements. On the other hand, the anonymity of sample is also a key question. 3. Rules: protocols and standard operating procedures (SOPs); The rules contain all the used protocols and procedures what are used in the laboratory activity from the receipt of sample to the release of result with the supporting tasks. The used analytical methods can be national or international standard method, method developed in approved institution or organization or own developed method (or house method). The first cases the validation of method adaption has to be performed, but in the case of house methods both validation and authorization are necessary. The documentation of test methods have to include the records of validation, the known limitations of the methods, the quality control of method and the methods of calibration. The modifications of procedures and protocols have to be kept marked with „outdated” or „revised” with the date of modification but the previous versions also have to be kept. New or modified methods have to be reaccredited. This section contains the rules on receipt, handling and storing of samples. It is declared that only authorized person can receive the sample. A documented marking system, combination of letters and numbers, has to be applied keeping the anonymity and providing an easier use. The storing conditions of samples have to fit the demands of sample (for example, if the nature of sample requires cooling or drying its circumstances have to be controlled) and they have to be prevented from unauthorized access. The samples have to be kept as long as it is necessary; generally the storage time is three months, but the rapidly deteriorating samples may be kept until the end of analysis. The samples have to be eliminated and distracted after storage under documented conditions. 4. Results Results are present on all stages of analysis as raw data, final report and archives. All data have to be kept during the analytical process (partial results, results and calculations) with all data necessary for traceability (method, date, name of analyst). All parts of result preparation have to be archived maintaining traceability. The partial results and final reports have to be collected in registered and numbered booklets, and collected and structured systematic collection of printed electronic files. The rules for the handling and storing of electronic records are separately listed. The laboratory has to provide confidential handling and storing of results. 5. Quality Assurance The quality assurance of the work of laboratory is an independent monitoring of analytical and research processes. For the quality assurance issues a quality manager or quality assurance unit, independent from the management of laboratory, is competent with the following tasks: • ensuring the compilance of GLP • handling and revising of documents containing the operation conditions 100 Created by XMLmind XSL-FO Converter. 15. Systems for products grading and quality certification laboratories • planning, organization, execution and documentation of training and retraining Laboratory accreditation is a certification that the laboratory has competence for a specific task. The accrediting organization has to be an independent and widely acknowledged one. It can be national (for example the Hungarian Accreditation Board – HAB (NAT) in Hungary) or international (as Grain and Feed Trade Association – GAFTA, or TÜV or United Kingdom Accreditation Service – UKAS). In the accreditation procedure the laboratory prepares for the certification. First, the laboratory has to get practice to carry out a specific, well-defined task (meet all the requirements on this field). The preparation also contains the development of quality assurance system. The Quality Manual (QM) have to be prepared with a structure follows the structure of the ISO/IEC 17025:2001. It has to contain the rules, procedures and methods used in the laboratory and prove the expertness (facilities, equipments, personnel and other aspects listed above). Then the laboratory starts to apply the QM and trains the employees to apply it in details. After acquiring the necessary proficiency, the laboratory requests to start the accreditation procedure by the accreditation organisation (AO). The first step of revision is the evaluation of QM by the experts of AO, the exploration of non-compliances and their indication, and they are corrected in the QM by the laboratory. After the acceptance of QM, the experts of AO perform a visit to the scene, verify the laboratory and its activities and prepare a report on the experiences. Based on the reports the Accreditation Board makes decision on the acceptance or rejection of the request. If the request is accepted the AO gives a reference number to the laboratory what have to be present on all the records and reports.The Laboratory also can perform other tests which are not accredited activities of it, but these results have to be marked by a comment, for example: „this is the result of a non-accredited test”. The accreditation is valid for a pre-defined time only. The base of laboratory quality assurance system and accreditation is the Quality Manual. Its general parts are the followings: 1. Description of the activities of the company and laboratory 2. Quality Policy Statement 3. Field of accreditation • types of the examined or analyzed products, materials or samples • types of the tests or analyses • list of the standards or methods, equipments, procedures indicating the concentration range and accuracy and precision 4. Description of the staff 5. Description of the facilities and equipments 6. Procedures 7. Validation 8. Sample handling 9. Results 10. Costumer service (feedbacks, complaint handling) 11. Handling of chemicals and wastes The ISO/IEC 17025:2001 standard requires more documentation. It is formed by three main parts: 1. Quality Management Manual The quality management manual is a public document available for all the potential costumers. It contains all the general information that makes ensuring possible about the structure and general regulations of quality assurance system, but it must not contain confidential information. 101 Created by XMLmind XSL-FO Converter. 15. Systems for products grading and quality certification laboratories 2. Quality Management Procedures The Quality Management Procedures is an internal documentation system containing the details of work of the laboratory, therefore it is a collection of specific regulations and in-depth description of processes. 3. Work Orders The Work Orders contains all the steps are necessary to perform the specific tasks. Validation is a part of the quality manual. It is a confirmation that the analytical method used by the laboratory meets the specific requirements for performing the analytical test (accuracy, adaptability, etc.). The validation must demonstrate the evidence of • the suitability of the analytical (testing) system / equipment • the suitability of the analytical (testing) method • the suitability of the testing laboratory and staff When the used method is standardized or previously validated one and the laboratory applies it without any change it do not have to be validated. Validation is necessary when it is developed by own or another laboratory and has not been validated yet, or it differs from the standard and the difference may affect the result (accuracy, precision and performance characteristics), or partial validation is required in the lack of chemicals, tools, equipment required by the standard, or the method is used for a sample different from the standard model matrix. The way and results of validation have to be record in a validation document with the following contents: • The requirements on analysis • the purpose of analysis • quantity requirements, concentration range of components to be determined • requirements on the analysis system • selectivity of the desired value • limit of determination • working range, • accuracy, • precision, • calculation from raw data to result • Results that prove suitability. • The detailed description of method (principles, scope of applicability, sampling and sample preparation, used chemicals, used tools, recipe-like description of the measurement, calculation and report of result, interfering factors) • Performance parameters (selectivity, measurement range, linearity, sensitivity, detection limit, quantification limit, immunity to interference, precision, uncertainty) 102 Created by XMLmind XSL-FO Converter. 16. fejezet - REFERENCES 113/2005.(X. 28.) MVH Közlemény a gabonafélék 2005/2006. gazdasági évben történő intervenciós felvásárlásáról 136/2011.(XII. 22.) VM rendelet a vágósertések vágás utáni minősítéséről és a hasított féltestek kereskedelmi osztályba sorolásáról 14/1998. (IV. 3.) FM rendelet a vágómarhák vágás utáni minősítéséről és a hasított féltestek kereskedelmi osztályba sorolásáról 16/1998. (IV. 3.) FM rendelet a vágójuhok vágás utáni minősítéséről és kereskedelmi osztályba sorolásáról 2003/858/EC Commission Decision of 21 November 2003 laying down the animal health conditions and certification requirements for imports of live fish, their eggs and gametes intended for farming, and live fish of aquaculture origin and products thereof intended for human consumption 76/2003.(VII. 4.)FVM rendelet a vágómarhák vágás utáni minősítéséről és a hasított féltestek kereskedelmi osztályba sorolásáról szóló 14/1998.(IV. 3.) FM rendelet módosításáról 9/2001. (III. 30.) EüM-FVM együttes rendelet a helyes laboratóriumi gyakorlat alkalmazásáról és ellenőrzéséről Aulenta F., Bassani C., Ligthart J.,Majone M,., Tilche A. 2002. Calorimetry: a tool for assessing microbial activity under aerobic and anoxic conditions. Water Research 36. 1297–1305 Baik, B:K:, Ullrich, S.E.: Barley for food: Characteristics, improvement, and renewed interest. Journal of Cereal Science 48 (2008) 233–242 Barta J. 2007: A gyümölcsfeldolgozás technológiái. In: A gyümölcsfeldolgozás technológiái (Szerk: Barta J.). Mezőgazda Kiadó, Budapest, 41-202. BeMiller J.N., Whistler R.L. (2009):Starch: Chemistry and Technology. Academic Press, 894.p. Bódi L. (2003) A baromfihús minősége – fogyasztói szempontok, mérési módszerek.A baromfi, I. 14.17. Brochers, R. (1962) A Note on the Digestibility of the Starch of High-Amylose Corn by Rats. Cereal Chem 39:145 – 146. CAC/RCP 58-2005: Code of hygienic practice http://www.codexalimentarius.org/download/standards/10196/CXP_058e.pdf for meat Christian, G.D. (2002): Analytical Chemistry 6th edition. Wiley, 848.p. Codex Alimentarius Hungaricus 1-3-1907/90: Certain marketing standards for eggs Codex Alimentarius Hungaricus 2-51: Dairy products Codex Alimentarius Hungaricus 1-3-103/76: Common marketing standards for certain fresh or chilled fishery products Codex Alimentarius Standard for Named Vegetable Oils (CODEX-STAN 210 ) Commission implementing regulation (EU) 543/2011 (7 June 2011) Commission Regulation (EC) No 1249/2008 of 10 December 2008 laying down detailed rules on the implementation of the Community scales for the classification of beef, pig and sheep carcases and the reporting of prices thereof Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs 103 Created by XMLmind XSL-FO Converter. REFERENCES Commission Regulation (EC) No 22/2008 of 11 January 2008 laying down detailed rules for the Community scale for the classification of carcases of ovine animals (Codified version) Commission Regulation (EU) No 420/2011 of 29 April 2011 amending Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs Commission Regulation (EC) No 790/2000 of 14 April 2000 laying down the marketing standard for tomatoes Commission Regulation (EC) No 824/2000 of 19 April 2000 establishing procedures for the taking-over of cereals by intervention agencies and laying down methods of analysis for determining the quality of cereals. Official Journal L 100 , 20/04/2000 P. 0031 – 0050 Commission Regulation (EC) No 660/2006 of 27 April 2006 Commission Regulation (EEC) No 1538/91 of 5 June 1991 introducing detailed rules for implementing Regulation (EEC) No 1906/90 on certain C1 marketing standards for poultrymeat Commission Regulation (EEC) No 920/89 of 10 April 1989 laying down quality standards for carrots, citrus fruit and dessert apples and pears and amending Commission Regulation No 58 Cornish, G. B., Békés, F., Allen H. M., Martin D. J. 2001.: Flour proteins ulinked to quality traits in an Australian doubled haploid wheat population. Australian Journal of Agricultural Research 52(12) 1339 – 1348 Council Regulation (EC) No 1234/2007 of 22 October 2007 establishing a common organisation of agricultural markets and on specific provisions for certain agricultural products (Single CMO Regulation) Council Regulation (EEC) No 1907/90 of 26 June 1990 on certain marketing standards for eggs Council Regulation (EEC) No 103/76 of 19 January 1976 laying down common marketing standards for certain fresh or chilled fish Csapó J., Albert Cs., Csapóné Kiss Zs. (2008): Élelmiszer analitika. Válogatott fejezetek. Scientia kiadó, Kolozsvár Csapó J., Csapóné Kiss Zs. (2004): Élelmiszerkémia. Mezőgazdasági Kiadó, Budapest Csapó J., Győri Z., Csapóné Kiss Zs., Borosné Győri A. (2008): Amino acid content of plant origin basic food and feed materials as a function of crude protein content. Acta Agraria Kaposváriensis 12. 3. 73-82. D’Apollonia, B. L.-W. H. Kunerth (1984): The Farinograph Handbook, AACC, St. Paul, Minnesota, USA. Davis, D., Nielsen, M.T. (1999).: Tobacco: Production, Chemistry, and Technology. Wiley-Blackwell, 467.p. Dióspatonyi I.: A húsfeldolgozás technológiája. http://www.chemonet.hu/hun/food/technol/husipar/husipar.html EC No. 8/1994: European Communities (beef carcase classification) regulations, 1994 Electronic Code of Federal Regulations - PART 810—official United States Standards For Grain FAO Agribusiness Handbook: Sugar beet white sugar". Food and Agriculture Organization, United Nations. 2009 FAO AGRICULTURAL SERVICES BULLETIN 150: Egg Marketing - A Guide for the Production and Sale of Eggs. http://www.fao.org/docrep/005/Y4628E/y4628e00.htm#Contents Faridi, H.-Rasper V. F.: (1987) The Alveograph Handbook, AACC, St. Paul, Minnesota USA Felföldi J. 2007: Gyümölcsök és gyümölcstermékek roncsolásmentes vizsgálati módszerei, eredményei. In: A gyümölcsfeldolgozás technológiái (Szerk: Barta J.). Mezőgazda Kiadó, Budapest, 248-256. Feng, P. (2007): Rapid methods for the detection of foodborne pathogens: Current and next-generation technologies. Food Microbiology, Fundamentals and frontiers, (Third edition) 911-934. 104 Created by XMLmind XSL-FO Converter. REFERENCES Fresh Fruits and Vegetables.First edition. World Health Organization, Food And Agriculture Organization Of The United Nations, Codex Alimentarius Commitee, Rome, 2007 Gasztonyi K. 2005: Amit a rozslisztek sütőipari értékéről tudni illik... Sütőiparosok, Pékek, 52. 15-21. p. Gasztonyi K.-Lásztity R. (szerk.) (1993): Élelmiszer-kémia 1. 2. Mezőgazda Kiadó, Budapest General guidelines on sampling - Codex Alimentarius Commission CAC/GL 50-2004 Goñi I., Díaz-Rubio M.E., Pérez-Jiménez J., Saura-Calixto F. (2009): Towards an updated methodology for measurement of dietary fiber, including associated polyphenols, in food and beverages. Food Research International, 42.7. 840–846 Győri Z. - Győriné Mile I. (2011): A búza és kukorica minősége és feldolgozása, Szaktudás Kiadó Ház Zrt., Budapest, Győri Z. (1999): A tápanyagellátás hatása a növények minőségére. In. Tápanyaggazdálkodás. 560-714. (Szerk.: Füleky Gy), Mezőgazda Kiadó, Budapest. Győri Z. 1995: Növényi termékek tárolása és feldolgozása. Egyetemi jegyzet, Debrecen, DATE Győriványi B. 1981.: Dohányipari technológia I-II. Mezőgazdasági Kiadó Budapest Győriványi B. 1986: A dohánygyártás alap- és segédanyagai. Mezőgazdasági Kiadó Budapest, Handbook: Good laboratory practice (2nd edition: Quality practices for regulated non-clinical research and development. World Health Organization 2009 http://www.who.int/tdr/publications/training-guidelinepublications/good-laboratory-practice-handbook/en/ Heinz, G., Hautzinger, P. 2007: Meat processing technology for small- to medium-scale producers. Food And Agriculture Organization Of The United Nations Regional Office For Asia And The Pacific Bangkok, ftp://ftp.fao.org/docrep/fao/010/ai407e Hoffmann, S. 2011: Ipari- és takarmánynövények termesztése. Debreceni Egyetem, Nyugat-Magyarországi Egyetem, Pannon Egyetem http://agritech.tnau.ac.in/seed/Seed_seedsampling.html http://mkk.szie.hu/dep/aeet/tanweb/termelet/tojas/tojas/text_tojas.htm http://people.umass.edu/~mcclemen/581Ash&Minerals.html http://people.umass.edu/~mcclemen/581Carbohydrates.html http://people.umass.edu/~mcclemen/581Moisture.html http://probes.invitrogen.com/resources/education/tutorials/4Intro_Flow/player.html http://rpa.defra.gov.uk/rpa/index.nsf/15f3e119d8abcb5480256ef20049b53a/f25f5c3daa27d08680257000003ef2 ba/$FILE/IM(C)15%20V4.0.pdf http://tgyi.fmk.nyme.hu/oktatas/bonyolultabb_mrses_ellenrzkrtyk_j.pdf http://vedyadhara.ignou.ac.in/wiki/index.php/PGDFSQM:Post_Graduate_Diploma_in_Food_Safety_and_Qualit y_Management/MVP-001:Food_Fundamentals_and_Chemistry http://www.123foodscience.com/ http://www.all-about-potatoes.com/ http://www.batscience.com/groupms/sites/BAT_7AWFH3.nsf/vwPagesWebLive/DO7AXG65?opendocument&SKN=1 105 Created by XMLmind XSL-FO Converter. REFERENCES http://www.cigarsupply.com/cigars/pc/Parts-of-the-Tobacco-Plant-and-Why-You-Should-Care-About-Themd23.htm http://www.codexalimentarius.net http://www.fao.org/docrep/004/T0279E/T0279E06.htm http://www.fao.org/docrep/T0395E/T0395E03.htm http://www.fao.org/docrep/t0567e/T0567E08.htm http://www.fao.org/docrep/T0818E/T0818E00.htm http://www.fao.org/docrep/x0039e/X0039E01.htm http://www.fas.usda.gov/psdonline/psdreport.aspx?hidReportRetrievalName=BVS&hidReportRetrievalID=533 &hidReportRetrievalTemplateID=5 http://www.inchem.org/documents/jecfa/jecmono/v47je05.htm#3.0 http://www.ltss.bris.ac.uk/jules/youngtrain/Water-Holding_Capacity%280-3%29/index.htm http://www.mansfield.ohio-state.edu/~sabedon/biol3010.htm#identification http://www.martinchrist.de/fileadmin/my_uploads/christ/en/Christ_Theorie_Katalog_en_web.pdf http://www.micquality.com/six_sigma_glossary/oc_curve.htm http://www.oecd.org/chemicalsafety/testingofchemicals/oecdseriesonprinciplesofgoodlaboratorypracticeglpandc ompliancemonitoring.htm http://www.thecattlesite.com/articles/685/nutrition-changes-milk-composition http://www.thepoultrysite.com/ourbooks/1/egg-quality-handbook/ http://www.unece.org/trade/agr/standard/meat/meat_e.html http://www.unece.org/trade/agr/welcome.html http://www.uq.edu.au/_School_Science_Lessons/UNBiology4A.html#9.1.2.8 http://www.wheatflourbook.org http://www.writescience.com/RMT%20PDFs/Elsevier/eans%20wetdig.pdf http://zeus.nyf.hu/~tkgt/okse/elista08/elis0806.pdf Huff -Lonergan, E., Lonergan, S.M. 2005: Mechanisms of water-holding capacity of meat: The role of post mortem biochemical and structural changes. A review.Meat Science 71.194–204. International Standards for Fruit and Vegetables: Early and Ware Potatoes. OECD Organisation for Economic Co-operation, Development, 2009 / OECD Publishing Jandal, J.M. (1996): Comparative aspects of goat and sheep milk. Small Ruminant Research, 22. (1996) 177185. Jávor A., Szigeti J. 2011: Termékminősítés és termékhigiénia. Debreceni Egyetem, Nyugat-Magyarországi Egyetem, Pannon Egyetem K. Marhawa (2010): Control and Analysis For Food and Agricultural Products. Gene-Tech Books, New Delhi Kent, N. L. (1980): Technology of cereals with special reference to wheat. Pergamon Press, Oxford Kruppa J. 2007: A biológiai és ökológiai tényezők hatása a burgonyára. Agrárágazat, 8. 7. 14-16 106 Created by XMLmind XSL-FO Converter. REFERENCES Laboratory Quality Management System Handbook. http://www.who.int/ihr/publications/lqms/en/index.html World Health Organization 2011 Láng G. (1976): Szántóföldi növénytermesztés. Mezőgazdasági Kiadó, Budapest Lásztity R., Törley D. (1987): Élelmiszeranalitika I-II. Mezőgazdasági Kiadó, Budapest Leffingwell J. C. 1976: Nitrogen components of leaf and their relationship to smoking quality and aroma Recent Advances in Tobacco Science, 2, 1-31. Lopez E.C.: Intestinal flow of microbial protein and rumen undegradable protein in cattle fed corn distillers grains and solubles, with emphasis during lactation. Theses and Dissertations in Animal Science.University of Nebraska-Lincoln, 2012. Marhawa, K. (2010): Control and Analysis For Food and Agricultural Products. Gene-Tech Books, Maya Publishing House, New Delhi Milk Processing Guide Series Volume 2 Published by: http://www.fao.org/ag/againfo/resources/documents/MPGuide/mpguide2.htm FAO/TCP/KEN/6611 Project MSZ 16802:2001 Tobacco. Quality requirements and grades of cured leaves MSZ 6380:1982 Soya-beans for industrial purposes N. Shakuntala O. Manay (2001): Food: Facts And Principles. New Age International, 564 p. Nádasdi J., Nádasdi J.-né: MEZŐGAZDASÁGI SZAKMODUL http://mmfk.nyf.hu/min/mgmodul/21.htm National Nutrient Database for Standard Reference Release 25 OECD series on principles of good laboratory practice and compliance monitoring Number 1. OECD Principles on Good Laboratory Practice (as revised in 1997). OECD Paris, 1998 Ostojić-Andrić, D., Aleksić, S., Hristov, S., Novaković Z., Petrović, M.M., Nikšić D., Stanišić, N. 2012: Serbia in the implementation of SEUROP standard for beef carcass clasiffication: legislation, parametars and evaluation criteria (part A) Biotechnology in Animal Husbandry 28 (1), p 47- 58 Pátkai Gy. 2007: Gyümölcsök és gyümölcstermékek beltartalmi vizsgálata érzékszervi és analitikai módszerekkel. In: A gyümölcsfeldolgozás technológiái (Szerk: Barta J.). Mezőgazda Kiadó, Budapest, 258-265. Pomeranz Y. (1978): Wheat Chemistry and Technology. The AACC Inc., St. Paul Potatoes.Vegetable Inspection Manuals of the Canadian Food Inspection Agency.http://www.inspection.gc.ca/food/fresh-fruits-and-vegetables/quality-inspection/vegetable-inspectionmanuals/potatoes/eng/1303766067711/1303766265738 R.U.C.I.P. for Inter-European trade in potatoes Edition Applicable from 1 March 2012. RUCIP European Committee, Paris, 2012 Recommended Methods of Sampling for Pesticide Residues for the Determination of Compliance with MRLs Codex Alimentarius Commission CAC/GL 33.1999 Skoog, D.A., West, D.M., Holler, F.J., Crouch, S.R. (2013): Fundamentals of Analytical Chemistry. Cengage Learning, 1072. p. Sipos P, Tóth Á, Pongráczné Barancsi Á, Győri Z (2007) : A búzaliszt reológiai vizsgálata különböző módszerekkel. Élelmiszervizsgálati Közlemények 53:(3) pp. 145-155. Stégerné Máté M. 2007: A gyümölcsfeldolgozás nyersanyagai. In: A gyümölcsfeldolgozás technológiái (Szerk: Barta J.). Mezőgazda Kiadó, Budapest, 7-32. Szedljak I. 2011: Dohány eredetű nyersanyagok minőségének s felhasználhatóságának biokémiai és mikrobiológiai jellemzése. Doktori értekezés. Budapesti Corvinus Egyetem 107 Created by XMLmind XSL-FO Converter. REFERENCES Szigeti T., Turza S., Molnár P.: A GLP és az élelmiszervizsgáló laboratóriumok akkreditálása az MSZ EN ISO/IEC 17025:2001-es szabvány alapján. Élelmiszervizsgálati Közlemények 49.2. 88-114. (2003) Tanács L (2005): Élelmiszer-ipari nyersanyagismeret. Szaktudás Kiadó Ház, Budapest Thaipong K., Boonprako U., Crosby K., Cisneros-Zevallos L., Hawkins Byrne D.: Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. Journal of Food Composition and Analysis 19 (2006) 669–675. Tirczka I 2000: Magyarország ökológiai adottságainak elemzése a cukorrépa-termesztés szempontjából. Doktori értekezés, SZIE, Gödöllő U.S. Soy: International Buyers’ Guide Chapter Two: Quality Standards for U.S. Soybeans and Soy Products. U.S. Soybean Export Council.http://www.ussec.org/wp-content/uploads/2012/08/Chap2.pdf Understanding the Codex Alimentarius. FAO/WHO Rome, Italy, 1999 UNECE standard ECE/TRADE/308 Ovine meat carcases and cuts United Nations, New York and Geneva, 2007. UNECE standard ECE/TRADE/326 Bovine meat carcases and cuts Revised version. United Nations, New York and Geneva, 2007. UNECE standard ECE/TRADE/355 Chicken meat carcases and parts.United Nations, New York and Geneva, 2007. UNECE standard ECE/TRADE/369 Porcine meat carcases and cuts United Nations, New York and Geneva, 2008. UNECE standard FFV-36 concerning the marketing and commercial quality control of tomatoes United Nations, New York and Geneva, (2012) UNECE standard FFV-50 concerning the marketing and commercial quality control of apples. United Nations, New York and Geneva, (2011) UNECE standard FFV-52 concerning the marketing and commercial quality control of early and ware potatoes. United Nations, New York and Geneva, (2009) United States Standards for Soybeans.http://www.gipsa.usda.gov/fgis/standards/810soybean.pdf. Vadáné Kovács M. 1999: A húsminőség alapjai. DATE MK, Debrecen, 92 p. Wheelis, M. 2011. Principles of Modern Microbiology, Jones and Bartlett Publishers, 25-47 Winfield M.,Hall M., Paynter B. (2007): Milling oat and feed oat quality - what are the differences? Government of Wastern Australia, Department of Agriculture and Food Wrigley, C.W., Batey, I.L. (Eds.) (2010): Cereal Grains: Assessing and Managing Quality. Woodhead Publishing Ltd., Oxford www.omgk.hu/magyarelelmiszerk.htm Yang L., Bashir R. 2008. Electrical/electrochemical impedance for rapid detection of foodborne pathogenic bacteria Biotechnology Advances 26 135-150 108 Created by XMLmind XSL-FO Converter. 17. fejezet - SAMPLE QUESTIONS 1. What is the definition of quality? 2. What are the components of quality? 3. What are the elements of quality control process? 4. What kind of quality requirements can be listed? What is the difference between them? 5. Expound the basic sources of quality requirements and analytical methods! 6. What aspects have to be considered during the selection of analytical method? 7. What is the aim of sampling? 8. What are lot, bulk and primary sample? 9. Expound the different kinds of samplings! 10. What kinds of error types are present in the sampling and what are they mean? 11. Expound the process of sampling! 12. Expound some sampling tools! 13. How can we decrease the size of sample? What do we have to consider during this? 14. Why is the storage of samples important? 15. Expound the process of sensory evaluation! 16. What are the most common and important physical parameters of foods and feeds and how can be they evaluated? Why are their values important? 17. What is the principle of NIR spectroscopy? 18. What is rheology? What are the most frequent rheologic analytical methods? 19. What are water content and water activity and what are the differences and similarities amongst them? 20. What is the sorption isotherm and why it is important? 21. How can we determine the water content of a sample? 22. What are the most important chemical components of an agricultural sample? 23. How can we classify the proteins? 24. What are the typical nitrogen or protein concentrations of different samples? 25. Expound the methods of determination of protein content and composition! 26. What are the general properties of lipids? 27. Expound the methods of determination of lipid content and composition! 28. Expound the methods of determination of carbohydrate content (total and reducing sugars, starch)! 29. Expound the methods of determination of total and dietary fiber content! 30. Expound the methods of determination of ash content! 109 Created by XMLmind XSL-FO Converter. SAMPLE QUESTIONS 31. What are the most common methods of determination of the micro element content? 32. Expound the principle of emission spectroscopy! 33. Expound the most common methods of the determination of vitamins! 34. What are the principles of the determination of antioxidant activity? 35. How can be the enzyme activity determined? 36. How can be the toxin content of agricultural samples determined? 37. What are the main factors influencing the growth of microbes and how can be they classified by their demands? 38. What are the most important factors of sampling for microbiologic analysis? 39. What is the general scheme for microbiological analysis of foods? 40. Microscopic methods – types and characteristic 41. What are the main types of culture media? 42. What are the possibilities of serologic identification? 43. Expound the basic chemical composition of winter wheat and other cereals! 44. How can we classify the cereal proteins by their solubility? What is the importance of this classification? 45. What is gluten? How can we present the gluten quality? 46. What is starch? What are its parts and how they influence the end-use? 47. Why amylase activity is important? How can be it characterized? 48. What is floating number and why is it important? 49. Expound the main aspects of the classification of cereals (corn for food and feed use, rice, barley)! 50. What is β-glucan and what kind of cereals are the main sources of it? 51. What is DDGS and for what can it be used for? 52. What are the primary and secondary quality parameters of sugar beet and what is the difference between these groups? 53. What are the main physical parameters of sugar beet and why are they important? 54. Expound the chemical composition of sugar beet and the significance of the most important compounds! 55. What are the most important oil crops? What are their main chemical components and what are their ratios? 56. Expound the classification of oil crops by their fatty acid composition! 57. Expound the main quality parameters of rapeseed! 58. Expound the chemical composition of potatoes! 59. How can be the potatoes classified by their physical parameters and end-use? 60. What are the minimum requirements on potatoes? 110 Created by XMLmind XSL-FO Converter. SAMPLE QUESTIONS 61. What are the types and reasons for flesh browning? 62. Expound the types of tobacco! 63. Expound the chemical composition of tobacco! 64. Expound the physical parameters of tobacco influencing its quality! 65. Expound the chemical composition of fruits and vegetables and the importance of main compounds! 66. Expound the general requirements on FFV! 67. How can we determine the maturity stage of the fruits? 68. What are the minimum requirements of marking of FFVs? 69. Expound the rating of apples! 70. Expound the rating of tomatoes! 71. What are the main fields of meat qualification? 72. Expound the main tissues of the animal body and the meat! 73. What is the structure of muscle? 74. Expound the post mortem processes of meat and their importance in meat quality! 75. What are the main characteristics of normal, PSE and DFD meat types? 76. Expound the physical quality parameters of meat! 77. What are the main chemical components of the different meat kinds? 78. What are the ante mortem inspections? 79. What are hygienic requirements of meat inspection? 80. What are the main results of post mortem inspection? 81. Expound the main disorders of meat! 82. What are the minimum requirements on meat quality? 83. Expound the qualification of the different animal species (bovine, porcine, ovine and poultry)! 84. What are the main physical and chemical properties of milk? 85. What are the main requirements on the raw and heat treated milk? 86. What is milk acidity and why is it important? 87. Expound the structure of eggs! 88. What are the main physical and chemical properties of eggs? 89. What are the main defects of the eggs? 90. What are the main types of laboratories and how can be their work characterized? 91. What are the main quality assurance systems for laboratories? What are the differences between them? 92. Expound the basics of GLP! 111 Created by XMLmind XSL-FO Converter. SAMPLE QUESTIONS 93. What is laboratory accreditation and how it is performed? 94. Expound the Quality Manuals! 95. What method validation means and what have to be documented by the laboratory? 112 Created by XMLmind XSL-FO Converter.