Unit 3 Metals
advertisement
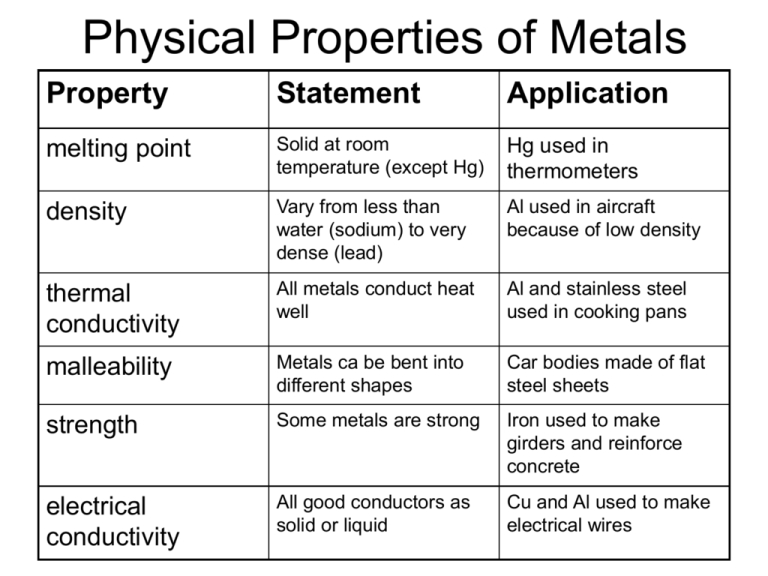
Physical Properties of Metals Property Statement Application melting point Solid at room temperature (except Hg) Hg used in thermometers density Vary from less than water (sodium) to very dense (lead) Al used in aircraft because of low density thermal conductivity All metals conduct heat well Al and stainless steel used in cooking pans malleability Metals ca be bent into different shapes Car bodies made of flat steel sheets strength Some metals are strong Iron used to make girders and reinforce concrete electrical conductivity All good conductors as solid or liquid Cu and Al used to make electrical wires Alloys The properties of metals can be improved by making them into metals. For example, aluminium is very lightweight and so would be ideal for making aeroplanes. However, it isn’t strong enough to hold passengers and their luggage. An alloy is formed when two or more metals or metals with non-metals are mixed together. Alloys are usually made by melting the mixture and then allowing it to cool until it has solidified. An alloy enhances the properties of a metal to make it more useful. Alnico (alloy made of aluminium, nickel and cobalt) keeps the lightweight property but adding the other metals makes it much stronger. Examples of Alloys Name of Alloy Metals present Uses brass bronze copper and zinc Door handles and fittings copper and tin statues solder tin and lead stainless steel Iron, chromium and nickel Soldering metals together Cutlery, kitchen sinks Reactivity of Metals • A list that places metals in order of their readiness to take part in chemical reactions • Reactive metals are at the top and unreactive metals are at the bottom • Reactions with oxygen, water and dilute acid can be used to put metals in order • The speed of a reaction can determine reactivity How do I remember the Reactivity Series • Use your data booklet – same as the Electrochemical Series except for the first 4. Remember the first 4 by using: Pupils potassium So Love Chemistry sodium lithium or calcium • Remember the following mnemonic: Please Send Lazy Charlie potassium sodium McLean A magnesium aluminium lithium calcium Zebra If zinc The iron tin Lean Horse Can’t Munch lead hydrogen* copper mercury Sweet Green Plants silver gold platinum * Hydrogen is a non metal but you will see why it is included in Unit 4B Metals as Resources • We obtain metals from rocks in the Earth known as metal ores. • An ore is a naturally occurring compound of a metal e.g iron ore contains iron oxide • Extraction involves obtaining a metal from its ore • Metals are often recycled as we have a finite (limited) supply • Unreactive metals can be found on Earth as the metals themselves. They are uncombined i.e have not formed compounds e.g gold and silver The date of discovery of a metal The cost of a metal The value of recycling a metal Can be related to two factors Whether it occurs naturally as an element or how easily it is extracted from its ores i.e how reactive it is How abundant (how plentiful) it is • Silver and gold have been known since earliest civilisation because they are unreactive and so were found uncombined • Aluminium and magnesium were not discovered until the nineteenth century since they are quite reactive and very difficult to extract from their ores • The cost of recycling aluminium is less than the cost of extracting aluminium from its ore Production of Iron from its Iron Ore • In industry, this is carried out in a blast furnace CO removes oxygen from iron ore Fe2O3 + 3CO bell valve coke 2Fe + 3CO2 waste gases CO2 reacts with carbon to form CO CO2 + C Coke burns in the blasts of hot air to form CO2 C + O2 iron ore, coke and limestone 2CO o 1500 C pre-heated air CO2 molten slag molten iron